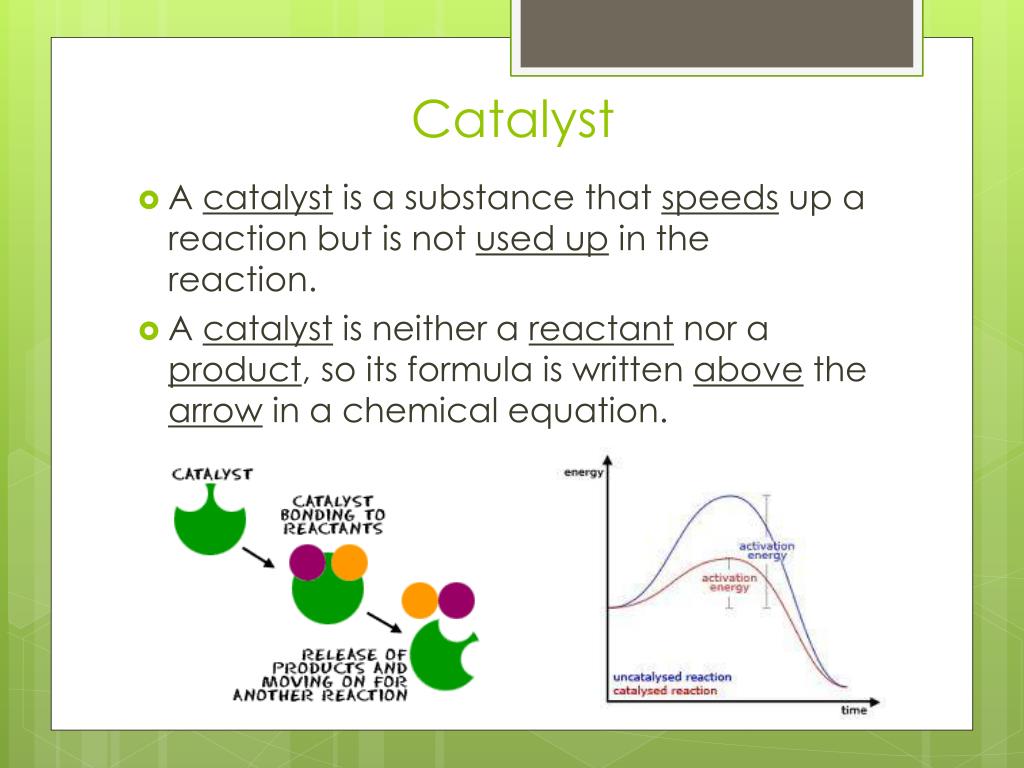
Catalyst Chemistry Equation . List examples of catalysis in. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. N 2 (g) + 3h 2 (g) 2nh 3 (g) so 2 (g) + o 2 (g) so 3 (g) different processes require different types of catalysts but they all work on the same principle: Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. By the end of this module, you will be able to: Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Explain the function of a catalyst in terms of reaction mechanisms and. Catalysts do not appear in the overall. They provide an alternative pathway for the reaction to occur. By the end of this section, you will be able to: Catalysts do not form part of the chemical equation but they are sometimes seen above or below the reaction arrow: A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction.
from www.slideserve.com
Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. They provide an alternative pathway for the reaction to occur. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts do not form part of the chemical equation but they are sometimes seen above or below the reaction arrow: By the end of this module, you will be able to: Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts do not appear in the overall. List examples of catalysis in.
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free
Catalyst Chemistry Equation Catalysts do not appear in the overall. Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. Catalysts participate in a chemical reaction and increase its rate. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. N 2 (g) + 3h 2 (g) 2nh 3 (g) so 2 (g) + o 2 (g) so 3 (g) different processes require different types of catalysts but they all work on the same principle: They do not appear in the reaction’s net equation and are not consumed during the reaction. List examples of catalysis in. Catalysts do not form part of the chemical equation but they are sometimes seen above or below the reaction arrow: Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. By the end of this module, you will be able to: By the end of this section, you will be able to: Explain the function of a catalyst in terms of reaction mechanisms and. Catalysts do not appear in the overall. They provide an alternative pathway for the reaction to occur. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts Catalyst Chemistry Equation List examples of catalysis in. By the end of this module, you will be able to: Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a. Catalyst Chemistry Equation.
From www.slideserve.com
PPT Mechanisms, Catalysts Intermediates and k PowerPoint Presentation Catalyst Chemistry Equation By the end of this section, you will be able to: Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. By the end of this module, you will be able to: Catalysts do not appear in the overall. Catalysts participate in a chemical reaction and increase its rate. Catalysts allow. Catalyst Chemistry Equation.
From www.youtube.com
Catalysts Chemistry Khan Academy YouTube Catalyst Chemistry Equation Catalysts participate in a chemical reaction and increase its rate. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. By the end of this section, you will be able to: Catalysts do not form part of the chemical equation but they are sometimes seen. Catalyst Chemistry Equation.
From www.slideserve.com
PPT §10.5 Catalytic reaction PowerPoint Presentation, free download Catalyst Chemistry Equation Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. List examples of catalysis in. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalysts do not appear in the overall. N 2 (g) +. Catalyst Chemistry Equation.
From exomdmjjp.blob.core.windows.net
Examples Of Catalysts Bbc Bitesize at Jacqueline Epp blog Catalyst Chemistry Equation They provide an alternative pathway for the reaction to occur. Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. Catalysts do not form part of the chemical equation but they are sometimes seen above or below the reaction arrow: Catalysts do not appear in the overall. Explain the function of. Catalyst Chemistry Equation.
From www.pinterest.com
Homogeneous Catalyst Easy Science Ap chemistry, Chemical equation Catalyst Chemistry Equation By the end of this module, you will be able to: They provide an alternative pathway for the reaction to occur. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. They do not appear in the reaction’s net equation and are not consumed during. Catalyst Chemistry Equation.
From hubpages.com
Chemical Reactions and Chemical Equations Owlcation Catalyst Chemistry Equation Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. They provide an alternative pathway for the reaction to occur. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalysts participate in a chemical reaction and increase its rate.. Catalyst Chemistry Equation.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent Catalyst Chemistry Equation They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Explain the function of a catalyst in terms of reaction mechanisms and. Catalysts in biological reactions are called enzymes close. Catalyst Chemistry Equation.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalyst Chemistry Equation Explain the function of a catalyst in terms of reaction mechanisms and. Catalysts do not form part of the chemical equation but they are sometimes seen above or below the reaction arrow: By the end of this section, you will be able to: Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a. Catalyst Chemistry Equation.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Chemistry Equation Catalysts do not appear in the overall. Explain the function of a catalyst in terms of reaction mechanisms and. By the end of this section, you will be able to: Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a substance that increases the rate of a. Catalyst Chemistry Equation.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalyst Chemistry Equation Catalysts do not form part of the chemical equation but they are sometimes seen above or below the reaction arrow: They do not appear in the reaction’s net equation and are not consumed during the reaction. List examples of catalysis in. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear. Catalyst Chemistry Equation.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation ID6634764 Catalyst Chemistry Equation By the end of this module, you will be able to: Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts do not form part of the chemical equation but they are sometimes seen above or below the reaction arrow: Catalysts in biological reactions are called enzymes close enzyme a. Catalyst Chemistry Equation.
From www.youtube.com
R2.2.6 Intermediates and catalysts (HL) YouTube Catalyst Chemistry Equation A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalysts participate in a chemical reaction and increase its rate. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts are substances that speed up. Catalyst Chemistry Equation.
From www.bartleby.com
Answered Identify the catalyst in each equation.… bartleby Catalyst Chemistry Equation They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts do not form part of the chemical equation but they are sometimes seen above or below the reaction arrow: Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation.. Catalyst Chemistry Equation.
From exozrdcct.blob.core.windows.net
Equations In Catalytic Converters at Adam Hassel blog Catalyst Chemistry Equation By the end of this module, you will be able to: Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. They provide an alternative pathway for the reaction to occur. By the end of this section, you will be able to: Catalysts are substances that speed up a reaction but which are not consumed. Catalyst Chemistry Equation.
From joilxpqmt.blob.core.windows.net
Chemical Catalysis Examples at Howard Wade blog Catalyst Chemistry Equation N 2 (g) + 3h 2 (g) 2nh 3 (g) so 2 (g) + o 2 (g) so 3 (g) different processes require different types of catalysts but they all work on the same principle: Catalysts participate in a chemical reaction and increase its rate. List examples of catalysis in. They provide an alternative pathway for the reaction to occur.. Catalyst Chemistry Equation.
From brainly.in
define catalyst with example ?? Brainly.in Catalyst Chemistry Equation Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. By the end of this module, you will be able to: Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. N 2 (g) + 3h 2 (g) 2nh 3 (g) so. Catalyst Chemistry Equation.
From joivqpwri.blob.core.windows.net
Catalyst In Chemistry Class 10 at Eric Dougherty blog Catalyst Chemistry Equation By the end of this section, you will be able to: Catalysts do not form part of the chemical equation but they are sometimes seen above or below the reaction arrow: Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. N 2 (g) + 3h 2 (g) 2nh 3 (g) so 2 (g) +. Catalyst Chemistry Equation.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalyst Chemistry Equation Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Explain the function of a catalyst in terms of reaction mechanisms and. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalysts are substances that. Catalyst Chemistry Equation.
From www.youtube.com
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube Catalyst Chemistry Equation Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a chemical reaction and increase its rate. Catalysts do not form part of the chemical equation but they are sometimes seen above. Catalyst Chemistry Equation.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Chemistry Equation Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. By the end of this module, you will be able to: By the end of this section, you will be able to: Catalysts do not appear in the overall. Catalysts allow a reaction to proceed via. Catalyst Chemistry Equation.
From ar.inspiredpencil.com
Catalyst Equation Catalyst Chemistry Equation N 2 (g) + 3h 2 (g) 2nh 3 (g) so 2 (g) + o 2 (g) so 3 (g) different processes require different types of catalysts but they all work on the same principle: Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. A catalyst is a substance that. Catalyst Chemistry Equation.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Catalyst Chemistry Equation N 2 (g) + 3h 2 (g) 2nh 3 (g) so 2 (g) + o 2 (g) so 3 (g) different processes require different types of catalysts but they all work on the same principle: Catalysts participate in a chemical reaction and increase its rate. Catalysts are substances that speed up a reaction but which are not consumed by it. Catalyst Chemistry Equation.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Chemistry Equation A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. By the end of this module, you will be able to: N 2 (g) + 3h 2 (g) 2nh 3 (g) so 2 (g) + o 2 (g) so 3 (g) different processes require different. Catalyst Chemistry Equation.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Chemistry Equation List examples of catalysis in. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. By the end of this module, you will be able to: Explain the function of a catalyst in terms of reaction mechanisms and. N 2 (g) + 3h 2 (g) 2nh 3 (g) so 2 (g). Catalyst Chemistry Equation.
From www.slideserve.com
PPT Chemical Equations & Reactions PowerPoint Presentation, free Catalyst Chemistry Equation Catalysts participate in a chemical reaction and increase its rate. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. List examples of catalysis in. N 2 (g) + 3h 2 (g) 2nh 3 (g) so 2 (g) + o 2 (g) so 3 (g) different processes require different types of catalysts but they all. Catalyst Chemistry Equation.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of Catalyst Chemistry Equation Catalysts do not form part of the chemical equation but they are sometimes seen above or below the reaction arrow: Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Explain the function of a catalyst in terms of reaction mechanisms and. Catalysts are substances that speed up a reaction but which are not consumed. Catalyst Chemistry Equation.
From loemotkul.blob.core.windows.net
What Is A Catalyst In A Chemical Reaction at Richard Starr blog Catalyst Chemistry Equation Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts do not form part of the chemical equation but they are sometimes seen above or below the reaction arrow: Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. They provide an alternative pathway for. Catalyst Chemistry Equation.
From www.tes.com
Secondary chemistry teaching resources Chemical reactions TES Catalyst Chemistry Equation Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. They provide an alternative pathway for the reaction to occur. By the end of this module, you will be able to: Catalysts participate in a chemical reaction and increase its rate. List examples of catalysis in. Catalysts are substances that speed up a reaction but. Catalyst Chemistry Equation.
From www.essentialchemicalindustry.org
Catalysis in industry Catalyst Chemistry Equation Catalysts do not form part of the chemical equation but they are sometimes seen above or below the reaction arrow: Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. By the end of this module,. Catalyst Chemistry Equation.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Catalyst Chemistry Equation Explain the function of a catalyst in terms of reaction mechanisms and. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. List examples of catalysis in.. Catalyst Chemistry Equation.
From loemotkul.blob.core.windows.net
What Is A Catalyst In A Chemical Reaction at Richard Starr blog Catalyst Chemistry Equation By the end of this section, you will be able to: By the end of this module, you will be able to: A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. They do not appear in the reaction’s net equation and are not consumed. Catalyst Chemistry Equation.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Chemistry Equation They do not appear in the reaction’s net equation and are not consumed during the reaction. By the end of this section, you will be able to: Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. Catalysts do not appear in the overall. Catalysts allow a reaction to proceed via. Catalyst Chemistry Equation.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Chemistry Equation By the end of this section, you will be able to: Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Catalysts do not form part of. Catalyst Chemistry Equation.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Chemistry Equation They do not appear in the reaction’s net equation and are not consumed during the reaction. By the end of this module, you will be able to: Catalysts participate in a chemical reaction and increase its rate. They provide an alternative pathway for the reaction to occur. List examples of catalysis in. Explain the function of a catalyst in terms. Catalyst Chemistry Equation.