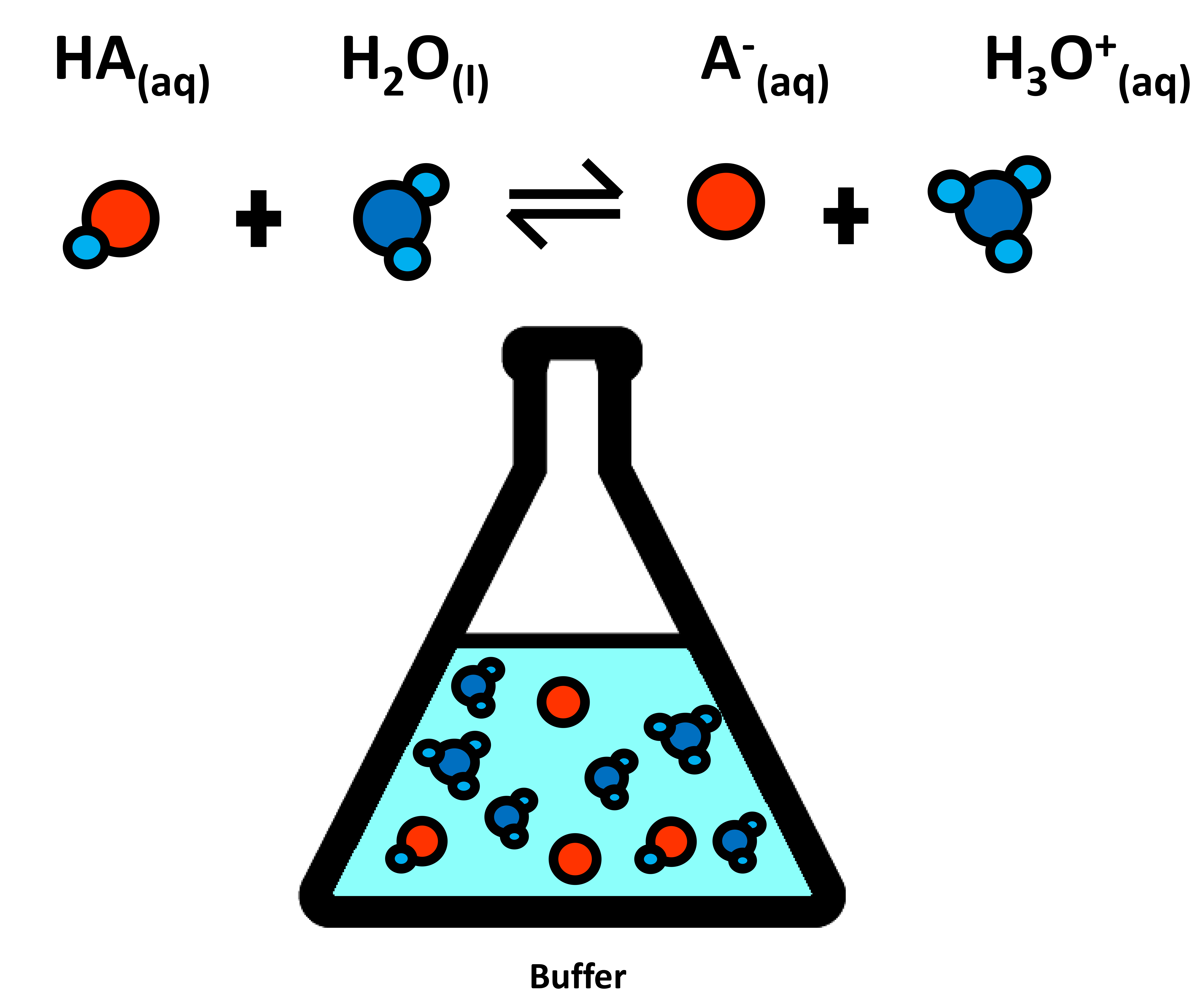
Buffer Lab Biology . Biological buffers are organic substances that help regulate the ph level in organisms. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. H + then reacts with water to form h 3 o +. They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. You should be aware that buffers play a critical role in almost all biochemical systems. Biochemical experiments routinely require a.
from www.vrogue.co
Biochemical experiments routinely require a. H + then reacts with water to form h 3 o +. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. Biological buffers are organic substances that help regulate the ph level in organisms. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. You should be aware that buffers play a critical role in almost all biochemical systems. They act by neutralizing excess hydrogen ions, thereby maintaining the.
How Do Buffers Work An Easy Explaination For Biologis vrogue.co
Buffer Lab Biology They act by neutralizing excess hydrogen ions, thereby maintaining the. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Biological buffers are organic substances that help regulate the ph level in organisms. Biochemical experiments routinely require a. You should be aware that buffers play a critical role in almost all biochemical systems. H + then reacts with water to form h 3 o +. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. They act by neutralizing excess hydrogen ions, thereby maintaining the.
From www.youtube.com
Buffer Experiment Concepts YouTube Buffer Lab Biology H + then reacts with water to form h 3 o +. Biological buffers are organic substances that help regulate the ph level in organisms. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions. Buffer Lab Biology.
From studylib.net
Buffers lab Buffer Lab Biology You should be aware that buffers play a critical role in almost all biochemical systems. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. Biochemical experiments routinely require a. They act by neutralizing excess hydrogen ions, thereby maintaining the. H + then reacts with water to form h 3 o. Buffer Lab Biology.
From www.vrogue.co
How Do Buffers Work An Easy Explaination For Biologis vrogue.co Buffer Lab Biology Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. You should be aware that buffers play a critical role in almost all biochemical systems. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. They act by neutralizing. Buffer Lab Biology.
From www.studocu.com
Experiment 2 Buffers and Buffer Capacity Experiment 2 Acids, Bases, and Buffers Buffer Lab Biology You should be aware that buffers play a critical role in almost all biochemical systems. They act by neutralizing excess hydrogen ions, thereby maintaining the. Biological buffers are organic substances that help regulate the ph level in organisms. Biochemical experiments routinely require a. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and. Buffer Lab Biology.
From www.studocu.com
Lab 3 report Laboratory N° 3 Biological Buffers IIPreparation of a Phosphate Buffer Lab Buffer Lab Biology Biological buffers are organic substances that help regulate the ph level in organisms. You should be aware that buffers play a critical role in almost all biochemical systems. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. The most relevant systems for biology are the carbonic acid/carbonate. Buffer Lab Biology.
From www.studocu.com
LAB 3 p H and Anatomy of a Buffer Biology Lab 2112 Lab 3 pH and Anatomy of a Buffer Studocu Buffer Lab Biology They act by neutralizing excess hydrogen ions, thereby maintaining the. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. Biochemical experiments routinely require a. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Biological buffers are organic. Buffer Lab Biology.
From www.labcompare.com
A Guide to Biological Buffer Preparation Buffer Lab Biology You should be aware that buffers play a critical role in almost all biochemical systems. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Study with quizlet and. Buffer Lab Biology.
From www.flinnsci.ca
Buffers in Household Products—Advanced Inquiry Laboratory Kit Flinn Scientific Buffer Lab Biology The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. H + then reacts with water to form h 3 o +. Biochemical experiments routinely require a. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. Biological buffers are organic substances. Buffer Lab Biology.
From www.youtube.com
pH and Buffer Part 3 Experimental Steps for Parts D and E YouTube Buffer Lab Biology You should be aware that buffers play a critical role in almost all biochemical systems. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Biological buffers are organic substances that help regulate the ph level in organisms. H + then reacts with water to form h 3. Buffer Lab Biology.
From www.avantorsciences.com
Buffers in Biology Lab Activity Educational Classroom Kits and Activities Buffer Lab Biology The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. You should be aware that buffers play a critical role in almost all biochemical systems. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. They act by neutralizing excess hydrogen ions,. Buffer Lab Biology.
From studylib.net
BUFFER LAB BIO H DR WEINER Introduction Buffers are Buffer Lab Biology Biological buffers are organic substances that help regulate the ph level in organisms. They act by neutralizing excess hydrogen ions, thereby maintaining the. You should be aware that buffers play a critical role in almost all biochemical systems. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids.. Buffer Lab Biology.
From www.youtube.com
TRU Chemistry Labs Experiment Titration Curves Part B Buffer Systems YouTube Buffer Lab Biology You should be aware that buffers play a critical role in almost all biochemical systems. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. They act by neutralizing. Buffer Lab Biology.
From www.scribd.com
Biology Lab 8 PDF PDF Acid Buffer Solution Buffer Lab Biology Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Biological buffers are organic substances that help regulate the ph level in organisms. They act by neutralizing excess hydrogen. Buffer Lab Biology.
From www.youtube.com
Lab 8 Acids, Bases, and Buffers experiment YouTube Buffer Lab Biology Biochemical experiments routinely require a. Biological buffers are organic substances that help regulate the ph level in organisms. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. You should be aware that buffers play a critical role in almost all biochemical systems. Buffers and how they control hydrogen ion concentrations,. Buffer Lab Biology.
From www.studocu.com
Lab 2 The scientific Method Buffer Lab 2 Buffers. Worth = (points earned/18)*15 = total Buffer Lab Biology H + then reacts with water to form h 3 o +. Biochemical experiments routinely require a. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. You should be aware that buffers play a critical role in almost all biochemical systems. The most relevant systems for biology. Buffer Lab Biology.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Buffer Lab Biology The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Biochemical experiments routinely require a. Biological buffers are organic substances that help regulate the ph level in organisms. They act by neutralizing excess hydrogen ions, thereby maintaining the. Study with quizlet and memorize flashcards containing terms like the measure of concentration. Buffer Lab Biology.
From studylib.net
Buffer Lab Procedure and Analysis Questions Buffer Lab Biology The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Biological buffers are organic substances that help regulate the ph level in organisms. Biochemical experiments routinely require a. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. They. Buffer Lab Biology.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies Buffer Lab Biology They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. You should be aware that buffers play a critical role in almost all biochemical systems. Biological buffers are organic substances that help regulate the ph level in organisms.. Buffer Lab Biology.
From www.docsity.com
Acid Base Buffers Lab Experiment 3 General Biology BIOL 1005 Docsity Buffer Lab Biology Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. You should be aware that buffers play a critical role in almost all biochemical systems. Biological buffers are organic substances that help regulate the ph level in organisms. H + then reacts with water to form h 3. Buffer Lab Biology.
From www.youtube.com
Buffer Lab HD 720p YouTube Buffer Lab Biology Biological buffers are organic substances that help regulate the ph level in organisms. You should be aware that buffers play a critical role in almost all biochemical systems. H + then reacts with water to form h 3 o +. Biochemical experiments routinely require a. They act by neutralizing excess hydrogen ions, thereby maintaining the. Study with quizlet and memorize. Buffer Lab Biology.
From www.studocu.com
Bio 1120 Lab 2 concentration, ph and buffers Khushi Koli EXPERIMENT 2 CONCENTRATION, pH Buffer Lab Biology The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. You should be aware that buffers play a critical role in almost all biochemical systems. Biochemical experiments routinely require a. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. They act. Buffer Lab Biology.
From www.studocu.com
Lab result Lab of a buffer Results Results The following tables demonstrate the results of Buffer Lab Biology Biochemical experiments routinely require a. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. H + then reacts with water to form h 3 o +. You should be aware that buffers play a critical role in almost all biochemical systems. Biological buffers are organic substances that help regulate the. Buffer Lab Biology.
From www.brainkart.com
Buffers Buffer Lab Biology You should be aware that buffers play a critical role in almost all biochemical systems. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. H + then reacts with water to form h 3 o +. Biochemical experiments routinely require a. They act by neutralizing excess hydrogen. Buffer Lab Biology.
From www.youtube.com
Buffers and pH Biology YouTube Buffer Lab Biology The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Biochemical experiments routinely require a. H + then reacts with water to form h 3 o +. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. Biological buffers are organic substances. Buffer Lab Biology.
From studylib.net
Lab 19 Buffers Buffer Lab Biology The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. They act by neutralizing excess hydrogen ions, thereby maintaining the. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. Biological buffers are organic substances that help regulate the ph level in. Buffer Lab Biology.
From www.youtube.com
WCLN Buffers Biology YouTube Buffer Lab Biology Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. H + then reacts with water to form h 3 o +. You should be aware that buffers play a critical role in almost all biochemical systems. Study with quizlet and memorize flashcards containing terms like the measure. Buffer Lab Biology.
From studylib.net
Buffer Lab march intake Buffer Lab Biology You should be aware that buffers play a critical role in almost all biochemical systems. Biological buffers are organic substances that help regulate the ph level in organisms. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. H + then reacts with water to form h 3 o +. Biochemical. Buffer Lab Biology.
From www.stellarscientific.com
Life Science Buffers for Molecular Biology Applications Lab Supplies Stellar Scientific Buffer Lab Biology H + then reacts with water to form h 3 o +. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Biological buffers are organic substances that help. Buffer Lab Biology.
From www.researchgate.net
(PDF) Buffer Biology Buffer Lab Biology Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Biological buffers are organic substances that help regulate the ph level in organisms. Biochemical experiments routinely require a. They act by neutralizing excess hydrogen ions, thereby maintaining the. Study with quizlet and memorize flashcards containing terms like the. Buffer Lab Biology.
From deporecipe.co
10x Protein Running Buffer Recipe Deporecipe.co Buffer Lab Biology Biological buffers are organic substances that help regulate the ph level in organisms. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. They act by neutralizing excess hydrogen ions, thereby maintaining. Buffer Lab Biology.
From www.studocu.com
Lab 3 p H and Anatomy of a Buffer Student Instructions Biology 1112/2112/2912 Lab 3 pH Buffer Lab Biology Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Biochemical experiments routinely require a. Biological buffers are organic substances that help regulate the ph level in organisms. H. Buffer Lab Biology.
From studylib.net
Buffer Lab Buffer Lab Biology Biological buffers are organic substances that help regulate the ph level in organisms. They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. H + then reacts with water to form h 3 o +. The most relevant. Buffer Lab Biology.
From www.vernier.com
Buffer Investigations > Experiment 1 from Investigating Biology through Inquiry Buffer Lab Biology You should be aware that buffers play a critical role in almost all biochemical systems. H + then reacts with water to form h 3 o +. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role. Buffer Lab Biology.
From www.coursehero.com
Buffers Experiment (dry lab) Determine the buffering capacity of a... Course Hero Buffer Lab Biology H + then reacts with water to form h 3 o +. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. They act by neutralizing excess hydrogen ions,. Buffer Lab Biology.
From www.studocu.com
Chemistry of Life p H and Buffers Chemistry of Life pH and Buffers Annalise Miller 1/11/ Data Buffer Lab Biology Biochemical experiments routinely require a. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. They act by neutralizing excess hydrogen ions, thereby maintaining the. Study with quizlet and memorize flashcards containing terms like the measure of concentration of hydrogen ions (h+) in solution.,. H + then reacts with water to. Buffer Lab Biology.