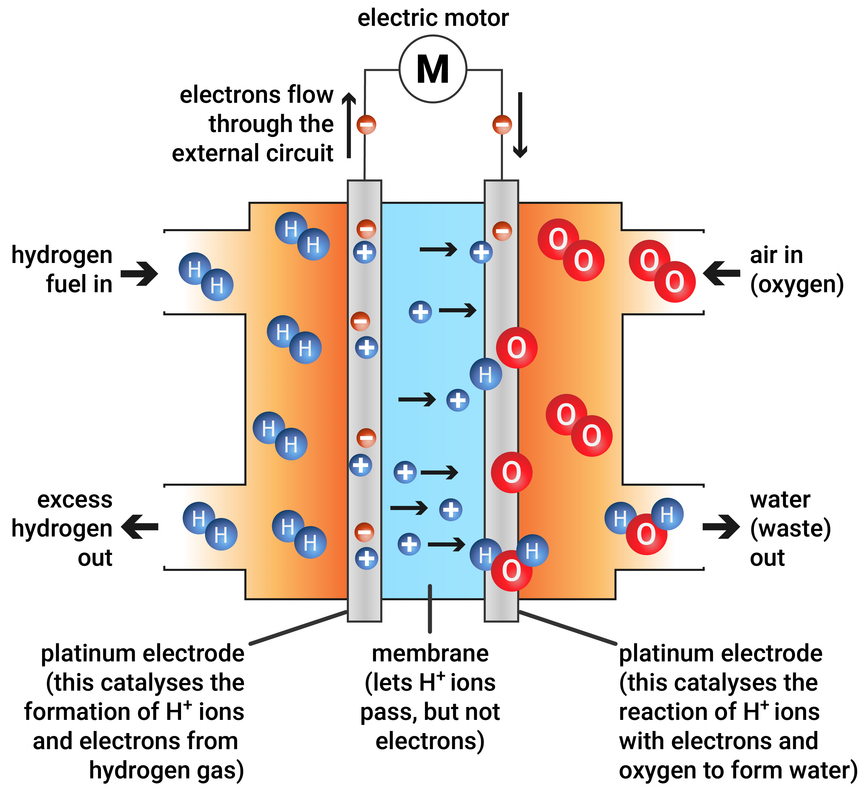
Fuel Cell Reaction Example . Classify batteries as primary or secondary. Gibbs free energy & voltage. List some of the characteristics and limitations of batteries. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. The (chemical) energy content of any fuel is called heating value (for. In this section, we describe the chemistry behind. By contrast to a conventional cell, where only limited quantities of oxidizing. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. The overall equation for the. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity.
from revisechemistry.uk
In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. Classify batteries as primary or secondary. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. By contrast to a conventional cell, where only limited quantities of oxidizing. Gibbs free energy & voltage. List some of the characteristics and limitations of batteries. The overall equation for the. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. In this section, we describe the chemistry behind. The (chemical) energy content of any fuel is called heating value (for.
Chemical Cells and Fuel Cells Edexcel T5 revisechemistry.uk
Fuel Cell Reaction Example Gibbs free energy & voltage. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. Classify batteries as primary or secondary. Gibbs free energy & voltage. List some of the characteristics and limitations of batteries. By contrast to a conventional cell, where only limited quantities of oxidizing. The (chemical) energy content of any fuel is called heating value (for. The overall equation for the. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. In this section, we describe the chemistry behind. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity.
From large.stanford.edu
Hydrogen Fuel Cells Fuel Cell Reaction Example The (chemical) energy content of any fuel is called heating value (for. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. Classify batteries as primary or secondary. The overall equation for the. By contrast to a conventional cell, where only limited quantities of oxidizing. In this. Fuel Cell Reaction Example.
From www.youtube.com
Activity 4 Modeling the Fuel Cell Reaction YouTube Fuel Cell Reaction Example By contrast to a conventional cell, where only limited quantities of oxidizing. Gibbs free energy & voltage. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. In this section, we describe the chemistry behind. A type of galvanic cell which promises to become increasingly important in the future is. Fuel Cell Reaction Example.
From www.researchgate.net
Design of a hybrid protonexchange membrane fuel cell Schematic of a... Download Scientific Fuel Cell Reaction Example Gibbs free energy & voltage. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. The (chemical) energy content of any fuel is called heating value (for. Classify batteries as primary or secondary. By contrast to a conventional cell, where only limited quantities of oxidizing. List some of the characteristics. Fuel Cell Reaction Example.
From www.slideserve.com
PPT Lecture 4 . Fuel Cell Reaction PowerPoint Presentation, free download ID2216845 Fuel Cell Reaction Example In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. Gibbs free energy & voltage. The (chemical) energy content of any fuel is called heating value (for. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction.. Fuel Cell Reaction Example.
From www.researchgate.net
Chemical reaction in proton exchange membrane fuel cell (PEMFC). Download Scientific Diagram Fuel Cell Reaction Example In this section, we describe the chemistry behind. The (chemical) energy content of any fuel is called heating value (for. Classify batteries as primary or secondary. List some of the characteristics and limitations of batteries. Gibbs free energy & voltage. By contrast to a conventional cell, where only limited quantities of oxidizing. A type of galvanic cell which promises to. Fuel Cell Reaction Example.
From opentextbc.ca
Applications of Redox Reactions Voltaic Cells Introductory Chemistry 1st Canadian Edition Fuel Cell Reaction Example A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. In this section, we describe the chemistry behind. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. List some of the characteristics and limitations of batteries. In contrast, a fuel cell. Fuel Cell Reaction Example.
From www.slideserve.com
PPT Lecture 4 . Fuel Cell Reaction PowerPoint Presentation, free download ID2216845 Fuel Cell Reaction Example In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. The (chemical) energy content of any fuel is called heating value (for. Classify batteries as primary or secondary. Gibbs free energy & voltage. A type of galvanic cell which promises to become increasingly important in the future. Fuel Cell Reaction Example.
From siqens.de
Principle, types, advantages What is a fuel cell? SIQENS Fuel Cell Reaction Example Gibbs free energy & voltage. The overall equation for the. In this section, we describe the chemistry behind. List some of the characteristics and limitations of batteries. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. A fuel cell can be defined as an electrochemical cell that generates electrical energy from. Fuel Cell Reaction Example.
From 640orfree.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip (2022) Fuel Cell Reaction Example A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. By contrast to a conventional cell, where only limited quantities of oxidizing. List some of the characteristics and limitations of batteries. In this section, we describe the chemistry behind. The overall equation for the. A type of galvanic cell which. Fuel Cell Reaction Example.
From revisechemistry.uk
Chemical Cells and Fuel Cells Edexcel T5 revisechemistry.uk Fuel Cell Reaction Example The overall equation for the. Classify batteries as primary or secondary. Gibbs free energy & voltage. By contrast to a conventional cell, where only limited quantities of oxidizing. In this section, we describe the chemistry behind. The (chemical) energy content of any fuel is called heating value (for. List some of the characteristics and limitations of batteries. A type of. Fuel Cell Reaction Example.
From www.slideserve.com
PPT Electrochemical Energy Conversion using Fuel Cell Systems PowerPoint Presentation ID9172677 Fuel Cell Reaction Example By contrast to a conventional cell, where only limited quantities of oxidizing. In this section, we describe the chemistry behind. The overall equation for the. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. Gibbs free energy & voltage. A fuel cell can be defined as. Fuel Cell Reaction Example.
From chem.libretexts.org
17.5 Batteries and Fuel Cells Chemistry LibreTexts Fuel Cell Reaction Example Classify batteries as primary or secondary. List some of the characteristics and limitations of batteries. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. By contrast to a conventional cell, where only limited quantities of oxidizing. The (chemical) energy content of any fuel is called heating value (for. Gibbs free energy. Fuel Cell Reaction Example.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cell Reaction Example Gibbs free energy & voltage. The overall equation for the. By contrast to a conventional cell, where only limited quantities of oxidizing. List some of the characteristics and limitations of batteries. In this section, we describe the chemistry behind. Classify batteries as primary or secondary. In contrast, a fuel cell is a galvanic cell that requires a constant external supply. Fuel Cell Reaction Example.
From www.researchgate.net
1 Fuel Cell Reaction through and external circuit, thus creating... Download Scientific Diagram Fuel Cell Reaction Example A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. Classify batteries as primary or secondary. In this section, we describe the chemistry behind. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. The overall equation. Fuel Cell Reaction Example.
From www.youtube.com
Proton Exchange Membrane Fuel Cell, Introduction, Principle, Advantages & Disadvantages YouTube Fuel Cell Reaction Example List some of the characteristics and limitations of batteries. Gibbs free energy & voltage. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. Classify batteries as primary or secondary. In this section, we describe the chemistry behind. The overall equation for the. The (chemical) energy content. Fuel Cell Reaction Example.
From www.youtube.com
Hydrogen & Fuel Cells Reactions Chemistry FuseSchool YouTube Fuel Cell Reaction Example A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. The (chemical) energy content of any fuel is called heating value (for. Gibbs free energy & voltage. Classify batteries as primary or secondary. By contrast to a conventional cell, where only limited quantities of oxidizing. The overall equation for the.. Fuel Cell Reaction Example.
From www.youtube.com
How hydrogen fuel cell works Fuel Cell Technology Working principle YouTube Fuel Cell Reaction Example In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. Classify batteries as primary or secondary. The overall equation for the. Gibbs free energy & voltage. The (chemical) energy content of any fuel is called heating value (for. List some of the characteristics and limitations of batteries.. Fuel Cell Reaction Example.
From www.chegg.com
Solved Hydrogen Fuel Cell Fuel cells are electrochemical Fuel Cell Reaction Example List some of the characteristics and limitations of batteries. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. The overall equation for the. Classify batteries as primary or secondary. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate. Fuel Cell Reaction Example.
From www.thesciencehive.co.uk
Fuel Cells (AQA) — the science sauce Fuel Cell Reaction Example The (chemical) energy content of any fuel is called heating value (for. By contrast to a conventional cell, where only limited quantities of oxidizing. Gibbs free energy & voltage. The overall equation for the. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A type of galvanic cell which. Fuel Cell Reaction Example.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cell Reaction Example Classify batteries as primary or secondary. The (chemical) energy content of any fuel is called heating value (for. The overall equation for the. In this section, we describe the chemistry behind. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. List some of the characteristics and limitations of batteries. Gibbs free. Fuel Cell Reaction Example.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Fuel Cell Reaction Example In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. By contrast to a conventional cell, where only limited quantities of oxidizing. The (chemical) energy content of any fuel is called heating value (for. A type of galvanic cell which promises to become increasingly important in the. Fuel Cell Reaction Example.
From www.researchgate.net
(a) Ethanolbased fuel cell reactions in different electrolytes.... Download Scientific Diagram Fuel Cell Reaction Example Gibbs free energy & voltage. By contrast to a conventional cell, where only limited quantities of oxidizing. The overall equation for the. In this section, we describe the chemistry behind. Classify batteries as primary or secondary. List some of the characteristics and limitations of batteries. The (chemical) energy content of any fuel is called heating value (for. In contrast, a. Fuel Cell Reaction Example.
From www.researchgate.net
(a) Ethanolbased fuel cell reactions in different electrolytes.... Download Scientific Diagram Fuel Cell Reaction Example A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. List some of the characteristics and limitations of batteries. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. Gibbs free energy & voltage. The (chemical) energy content of any fuel is. Fuel Cell Reaction Example.
From www.researchgate.net
Electrochemical reactions in a fuel cell with (A) H 2 O 2 and (B) CH 4... Download Scientific Fuel Cell Reaction Example By contrast to a conventional cell, where only limited quantities of oxidizing. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. In this section, we describe the chemistry behind. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate. Fuel Cell Reaction Example.
From studymind.co.uk
Fuel Cells (GCSE Chemistry) Study Mind Fuel Cell Reaction Example A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. The overall equation for the. List some of the characteristics and limitations of batteries. The (chemical) energy content of. Fuel Cell Reaction Example.
From nadeesharangikacarguy.blogspot.com
The Car Guy Hydrogen fuel cell technology for cars Fuel Cell Reaction Example A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. The (chemical) energy content of any fuel is called heating value (for. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. The overall equation for the. Gibbs free. Fuel Cell Reaction Example.
From www.researchgate.net
Oxidation reactions in fuel cells (cathodic and anodic half reactions... Download Table Fuel Cell Reaction Example In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical. Fuel Cell Reaction Example.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the reactions that occur at the Fuel Cell Reaction Example In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. Gibbs free energy & voltage. The (chemical) energy content of any fuel is called heating value (for. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction.. Fuel Cell Reaction Example.
From www.slideserve.com
PPT Fuel Cell Catalysts Based on Metal Nanoparticles PowerPoint Presentation ID2380024 Fuel Cell Reaction Example By contrast to a conventional cell, where only limited quantities of oxidizing. Classify batteries as primary or secondary. The (chemical) energy content of any fuel is called heating value (for. The overall equation for the. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. In contrast, a fuel cell. Fuel Cell Reaction Example.
From www.researchgate.net
Schematic representation of Direct Methanol Fuel Cell (DMFC). This fuel... Download Scientific Fuel Cell Reaction Example In this section, we describe the chemistry behind. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. The overall equation for the. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. Gibbs free energy & voltage. The. Fuel Cell Reaction Example.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cell Reaction Example In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. By contrast to a conventional cell, where only limited quantities of oxidizing. The (chemical) energy content of any fuel is called heating value (for. Gibbs free energy & voltage. The overall equation for the. In this section,. Fuel Cell Reaction Example.
From www.myelectrical2015.com
Electrical Revolution Working Principle of Fuel Cell Fuel Cell Reaction Example A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. The overall equation for the. By contrast to a conventional cell, where only limited quantities of oxidizing. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity.. Fuel Cell Reaction Example.
From www.researchgate.net
Schematic representation of a molten carbonate fuel cell (MCFC).... Download Scientific Diagram Fuel Cell Reaction Example List some of the characteristics and limitations of batteries. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. Gibbs free energy & voltage. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. In this section, we describe the chemistry behind.. Fuel Cell Reaction Example.
From www.slideserve.com
PPT How Fuel Cells Work PowerPoint Presentation, free download ID1700278 Fuel Cell Reaction Example A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. List some of the characteristics and limitations of batteries. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. A type of galvanic cell which promises to. Fuel Cell Reaction Example.
From www.researchgate.net
Scheme of a solid oxide fuel cell. Download Scientific Diagram Fuel Cell Reaction Example The overall equation for the. In this section, we describe the chemistry behind. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. By contrast to a conventional cell,. Fuel Cell Reaction Example.