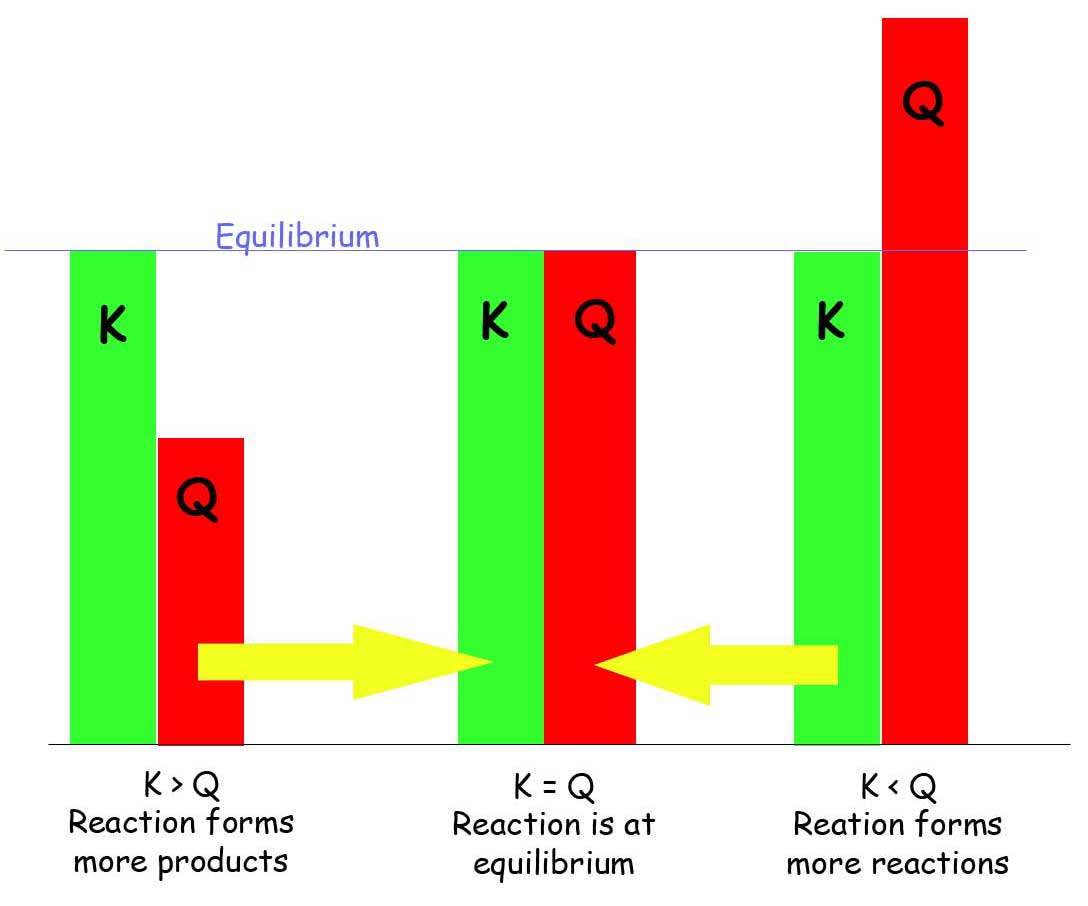
Equilibrium K Equation . the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. for a system at equilibrium, the law of mass action relates \(k\) to the ratio of the equilibrium concentrations of. Understanding the equilibrium constant () is crucial for.
from chem.libretexts.org
the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. for a system at equilibrium, the law of mass action relates \(k\) to the ratio of the equilibrium concentrations of. Understanding the equilibrium constant () is crucial for. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction.
Gas Equilibrium Constants Chemistry LibreTexts
Equilibrium K Equation Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. for a system at equilibrium, the law of mass action relates \(k\) to the ratio of the equilibrium concentrations of. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. Understanding the equilibrium constant () is crucial for. the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants;
From lessonlibundertoned.z22.web.core.windows.net
How To Write An Equilibrium Constant Equation Equilibrium K Equation the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. Explain why there may be an infinite number of values for the reaction quotient of a. Equilibrium K Equation.
From haipernews.com
How To Calculate Equilibrium Constant K Haiper Equilibrium K Equation for a system at equilibrium, the law of mass action relates \(k\) to the ratio of the equilibrium concentrations of. the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. . Equilibrium K Equation.
From www.youtube.com
How to Write Equilibrium Constant Expression (K, Keq, Kc, Kp) Practice Equilibrium K Equation for a system at equilibrium, the law of mass action relates \(k\) to the ratio of the equilibrium concentrations of. Understanding the equilibrium constant () is crucial for. a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. the equilibrium constant,. Equilibrium K Equation.
From materiallibdavenport.z4.web.core.windows.net
Equilibrium Constant K Equation Equilibrium K Equation Understanding the equilibrium constant () is crucial for. Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; the equilibrium constant, k, expresses the relationship between products and reactants of a. Equilibrium K Equation.
From www.chegg.com
Solved Identify the correct equation for the equilibrium Equilibrium K Equation for a system at equilibrium, the law of mass action relates \(k\) to the ratio of the equilibrium concentrations of. Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. Understanding the equilibrium constant () is crucial for. a reaction exhibiting a large k will reach equilibrium when most. Equilibrium K Equation.
From www.bartleby.com
Equilibrium Equations bartleby Equilibrium K Equation Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. Understanding the equilibrium constant () is crucial for. the k eq and k. Equilibrium K Equation.
From artofsmart.com.au
Guide to HSC Chemistry Module 5 Equilibrium and Acid Reactions Equilibrium K Equation Understanding the equilibrium constant () is crucial for. the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Explain why there may be an infinite number of values for the reaction quotient of a. Equilibrium K Equation.
From www.slideserve.com
PPT Equilibrium Constant (K eq ) PowerPoint Presentation, free Equilibrium K Equation Understanding the equilibrium constant () is crucial for. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; for a system at equilibrium, the law of mass action relates \(k\) to the ratio. Equilibrium K Equation.
From www.youtube.com
How To Calculate The Equilibrium Constant Kc Practice Problems Equilibrium K Equation the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; Understanding the equilibrium constant () is crucial for. Explain why there may be an infinite number of values for the reaction quotient of a. Equilibrium K Equation.
From haipernews.com
How To Find Q Equilibrium Constant Haiper Equilibrium K Equation the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; Understanding the equilibrium constant () is crucial for. Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. the equilibrium constant, k, expresses the relationship between products and reactants of a. Equilibrium K Equation.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation Equilibrium K Equation a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. for a system at equilibrium, the law of mass action relates \(k\) to. Equilibrium K Equation.
From www.youtube.com
Week 13 10. Calculating an equilibrium constant, K, from cell Equilibrium K Equation the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. Understanding the equilibrium constant () is crucial for. Explain why there may be an. Equilibrium K Equation.
From www.vrogue.co
How To Write An Equilibrium Constant Equation vrogue.co Equilibrium K Equation Understanding the equilibrium constant () is crucial for. the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Explain why there may be an infinite number of values for the reaction quotient of a. Equilibrium K Equation.
From www.slideserve.com
PPT The Equilibrium Constant, K, and The Reaction Quotient, Q Equilibrium K Equation Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. Understanding the equilibrium constant () is crucial for. a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. the k eq and k. Equilibrium K Equation.
From haipernews.com
How To Calculate The Equilibrium Constant Haiper Equilibrium K Equation Understanding the equilibrium constant () is crucial for. a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; Explain why there may be an. Equilibrium K Equation.
From www.slideserve.com
PPT Chapter 14 Chemical Equilibrium PowerPoint Presentation, free Equilibrium K Equation the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; for a system at equilibrium, the law of mass action relates \(k\) to the ratio of the equilibrium concentrations of. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Understanding the equilibrium. Equilibrium K Equation.
From www.pinterest.com
K = The Equilibrium Constant. [A] = Molarity of A in the solution. Ap Equilibrium K Equation a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Explain why there may be an infinite number of values for the reaction quotient of a. Equilibrium K Equation.
From www.vrogue.co
Calculating Equilibrium Constant Kc A Level Chemistry vrogue.co Equilibrium K Equation a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. Understanding the equilibrium constant () is crucial for. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. for a system at equilibrium, the law. Equilibrium K Equation.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM Chapter 16 PowerPoint Presentation, free Equilibrium K Equation for a system at equilibrium, the law of mass action relates \(k\) to the ratio of the equilibrium concentrations of. Understanding the equilibrium constant () is crucial for. a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. Explain why there may. Equilibrium K Equation.
From printablemediaadrianna.z13.web.core.windows.net
How To Write An Equilibrium Constant Equation Equilibrium K Equation a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; the equilibrium constant, k, expresses the relationship between products and reactants of a. Equilibrium K Equation.
From www.researchgate.net
Reactions and equilibrium constant equations. Download Scientific Diagram Equilibrium K Equation the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; Understanding the equilibrium constant () is crucial for. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. for a system at equilibrium, the law of mass action relates \(k\) to the ratio. Equilibrium K Equation.
From quizdbpharmacies.z4.web.core.windows.net
How To Calculate Equilibrium Temperature Equilibrium K Equation a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; Explain why there may be an infinite number of values for the reaction quotient. Equilibrium K Equation.
From facts.net
13 Fascinating Facts About Equilibrium Constant (Kc) Equilibrium K Equation the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Understanding the equilibrium constant (). Equilibrium K Equation.
From learningchemistryeasily.blogspot.com
Learning Chemistry Easily Chemical Equilibrium Theory, An Introduction Equilibrium K Equation for a system at equilibrium, the law of mass action relates \(k\) to the ratio of the equilibrium concentrations of. a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. the equilibrium constant, k, expresses the relationship between products and reactants. Equilibrium K Equation.
From pametno21.blogspot.com
K Constant Formula Chemistry pametno Equilibrium K Equation the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; Understanding the equilibrium constant () is crucial for. Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. the equilibrium constant, k, expresses the relationship between products and reactants of a. Equilibrium K Equation.
From general.chemistrysteps.com
Kp Equilibrium Constant and Partial Pressure Chemistry Steps Equilibrium K Equation Understanding the equilibrium constant () is crucial for. Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. the k eq and k. Equilibrium K Equation.
From www.physicsforums.com
Solving Equilibrium Constant Equilibrium K Equation for a system at equilibrium, the law of mass action relates \(k\) to the ratio of the equilibrium concentrations of. Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. Understanding the equilibrium constant () is crucial for. the equilibrium constant, k, expresses the relationship between products and reactants. Equilibrium K Equation.
From chem.libretexts.org
Gas Equilibrium Constants Chemistry LibreTexts Equilibrium K Equation for a system at equilibrium, the law of mass action relates \(k\) to the ratio of the equilibrium concentrations of. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a. Equilibrium K Equation.
From www.youtube.com
[A] Equilibrium Using K YouTube Equilibrium K Equation for a system at equilibrium, the law of mass action relates \(k\) to the ratio of the equilibrium concentrations of. a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. Understanding the equilibrium constant () is crucial for. the equilibrium constant,. Equilibrium K Equation.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation Equilibrium K Equation a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; Understanding the equilibrium constant () is crucial for. for a system at equilibrium,. Equilibrium K Equation.
From classnotes.org.in
Characteristics Of Equilibrium Constant Chemistry, Class 11, Equilibrium Equilibrium K Equation the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. the k eq and k p expressions are formulated as amounts of products divided by amounts of reactants; a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k. Equilibrium K Equation.
From www.youtube.com
7.1 What Happens to Kc When the Equilibrium Equation Coefficients are Equilibrium K Equation a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Explain why there may be an infinite number of values for the reaction quotient of a. Equilibrium K Equation.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc Membership Equilibrium K Equation Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. Understanding the equilibrium constant () is crucial for. the k eq and k. Equilibrium K Equation.
From www.youtube.com
Keq Equilibrium Constant (EVERYTHING YOU NEED TO KNOW CHEMISTRY) YouTube Equilibrium K Equation for a system at equilibrium, the law of mass action relates \(k\) to the ratio of the equilibrium concentrations of. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Explain why there may be an infinite number of values for the reaction quotient of a reaction at a. Understanding the equilibrium. Equilibrium K Equation.
From www.pinterest.es
an image of calculations for the equilibum cantant k2c Equilibrium K Equation a reaction exhibiting a large k will reach equilibrium when most of the reactant has been converted to product, whereas a small k indicates the reaction. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. the k eq and k p expressions are formulated as amounts of products divided by. Equilibrium K Equation.