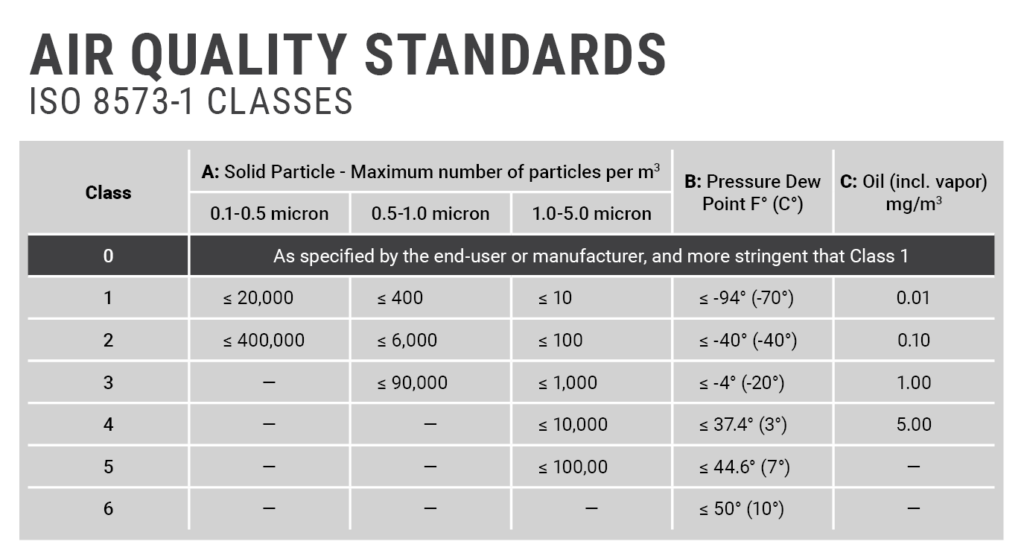
Usp Requirements For Compressed Air . The usp (the us pharmacopoeia) handle the testing of water or oil content quite easily: Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. For compressed air applications outside the clean room, the quality requirements are less clearly defined. Compressed air validation including tests like determination of moisture content, presence of oil content and microbial evaluation. Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can properly evaluate the quality of their processed gases including. The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. A reference point for the selection of. National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach.
from jhfoster.com
Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. The usp (the us pharmacopoeia) handle the testing of water or oil content quite easily: Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can properly evaluate the quality of their processed gases including. Compressed air validation including tests like determination of moisture content, presence of oil content and microbial evaluation. The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. For compressed air applications outside the clean room, the quality requirements are less clearly defined. A reference point for the selection of.
Ensure Compressed Air Purity Levels Using ISO JHFOSTER
Usp Requirements For Compressed Air Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. A reference point for the selection of. For compressed air applications outside the clean room, the quality requirements are less clearly defined. The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can properly evaluate the quality of their processed gases including. The usp (the us pharmacopoeia) handle the testing of water or oil content quite easily: Compressed air validation including tests like determination of moisture content, presence of oil content and microbial evaluation. Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1.
From www.drugs.com
Air Compressed Package Insert Usp Requirements For Compressed Air National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. A reference point for the selection of. Compressed air validation including tests like determination of moisture content, presence of oil content and microbial evaluation. The validation of a compressed air system in the pharmaceutical industry is typically performed. Usp Requirements For Compressed Air.
From www.drugtopics.com
USP Updated Guidelines Usp Requirements For Compressed Air Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. For compressed air applications outside the clean room, the quality requirements are less clearly defined. A reference point for the selection of. The usp (the us pharmacopoeia) handle the testing of water or oil content. Usp Requirements For Compressed Air.
From www.ifsqn.com
Compressed Air How Clean is Yours? International Food Safety and Usp Requirements For Compressed Air Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can properly evaluate the quality of their processed gases including. Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. National standards bodies have guidance documents for compressed air sampling, and reference is. Usp Requirements For Compressed Air.
From airpro.com.pk
Complete Compressed Air Installations Usp Requirements For Compressed Air Compressed air validation including tests like determination of moisture content, presence of oil content and microbial evaluation. Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. For compressed air applications outside the clean room, the quality requirements are less clearly defined. National standards bodies. Usp Requirements For Compressed Air.
From www.industrialair.co.nz
Compressed air quality standards Usp Requirements For Compressed Air Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. For compressed air applications outside the clean room, the quality requirements are less clearly defined. Compressed air validation including tests like determination of moisture content, presence of oil content and microbial evaluation. The usp (the. Usp Requirements For Compressed Air.
From studylib.net
USP Reference Standards Catalog Usp Requirements For Compressed Air A reference point for the selection of. Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. National standards. Usp Requirements For Compressed Air.
From www.usp.org
Reference Standards USP Usp Requirements For Compressed Air The usp (the us pharmacopoeia) handle the testing of water or oil content quite easily: Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can properly evaluate the quality of their processed. Usp Requirements For Compressed Air.
From www.nexflow.com
Importance of Compressed Air for Medical Use in Hospitals Nex Flow Usp Requirements For Compressed Air The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. A reference point for the selection of. National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. Labeling— where it is piped. Usp Requirements For Compressed Air.
From www.setra.com
Best Practices for USP and Compliance Usp Requirements For Compressed Air The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. Compressed air validation including tests like determination of moisture content, presence of oil content and microbial evaluation. Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can properly evaluate the quality. Usp Requirements For Compressed Air.
From dailymed.nlm.nih.gov
Oxygen Compressed USP Usp Requirements For Compressed Air Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. A reference point for the selection of. For compressed air applications outside the clean room, the quality requirements are less clearly defined. Compressed air validation including tests like determination of moisture content, presence of oil. Usp Requirements For Compressed Air.
From dailymed.nlm.nih.gov
Oxygen, USP Usp Requirements For Compressed Air Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can properly evaluate the quality of their processed gases including. For compressed air applications outside the clean room, the quality requirements are less clearly defined. A reference point for the selection of. Labeling— where it is piped directly from the collecting tank to the point of use, label. Usp Requirements For Compressed Air.
From www.youtube.com
FMS 5 Handling of Medical Gases and Compressed Air YouTube Usp Requirements For Compressed Air Compressed air validation including tests like determination of moisture content, presence of oil content and microbial evaluation. A reference point for the selection of. Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. Using the guidance of the us fda and ispe gpg, pharmaceutical. Usp Requirements For Compressed Air.
From dailymed.nlm.nih.gov
OXYGEN COMPRESSED USP LABEL Usp Requirements For Compressed Air Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can properly evaluate the quality of their processed gases including. The validation of a compressed air system in the pharmaceutical industry is typically. Usp Requirements For Compressed Air.
From www.hafner-pneumatik.com
The General Design of a Pneumatic System and its Components Usp Requirements For Compressed Air National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. Compressed air validation including tests like determination of moisture content, presence of. Usp Requirements For Compressed Air.
From www.fagron.us
Regulatory Update USP 795 and 797 Fagron, Inc. Usp Requirements For Compressed Air A reference point for the selection of. The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. The usp (the us pharmacopoeia). Usp Requirements For Compressed Air.
From www.airchecklab.com
Compressed Air System Risk Assessment Do I Need to Test? Trace Usp Requirements For Compressed Air Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can properly evaluate the quality of their processed gases including. Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. The usp (the us pharmacopoeia) handle the testing of water or oil content. Usp Requirements For Compressed Air.
From jhfoster.com
Ensure Compressed Air Purity Levels Using ISO JHFOSTER Usp Requirements For Compressed Air Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. The usp (the us pharmacopoeia) handle the testing of water or oil content quite easily: A reference point for the selection of. Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can. Usp Requirements For Compressed Air.
From www.pinterest.at
Compressed Air Top 10 Safety Posters Promote Safety Safety posters Usp Requirements For Compressed Air A reference point for the selection of. For compressed air applications outside the clean room, the quality requirements are less clearly defined. Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can. Usp Requirements For Compressed Air.
From www.processsensing.com
Applications for Industrial Dew Point Sensors Usp Requirements For Compressed Air Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can properly evaluate the quality of their processed gases including. Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. A reference point for the selection of. Compressed air validation including tests like. Usp Requirements For Compressed Air.
From www.airchecklab.com
How to Designate ISO 85731 Purity Classes Trace Analytics, the Usp Requirements For Compressed Air The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. For compressed air applications outside the clean room, the. Usp Requirements For Compressed Air.
From blog.spongejet.com
Air Compressor Requirements for Sandblasting Usp Requirements For Compressed Air A reference point for the selection of. The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. The usp (the us pharmacopoeia) handle the testing of water or oil content quite easily: For compressed air applications outside the clean room, the quality requirements are. Usp Requirements For Compressed Air.
From www.industrialair.co.nz
Compressed Air Requirements Free Guideline IAS Usp Requirements For Compressed Air A reference point for the selection of. Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can properly evaluate the quality of their processed gases including. The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. The usp (the us pharmacopoeia). Usp Requirements For Compressed Air.
From www.industrialoutpost.com
Compressed Air Requirements Chart Industrial Outpost The Official Usp Requirements For Compressed Air National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can properly evaluate the quality of their processed gases including. Compressed air validation including tests like determination of moisture content, presence of oil content and microbial. Usp Requirements For Compressed Air.
From dailymed.nlm.nih.gov
Oxygen Compressed USP Usp Requirements For Compressed Air The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. A reference point for the selection of. The usp (the us pharmacopoeia) handle the testing of water or oil content quite easily: For compressed air applications outside the clean room, the quality requirements are. Usp Requirements For Compressed Air.
From dailymed.nlm.nih.gov
AIR USP COMPRESSED Usp Requirements For Compressed Air National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can properly evaluate the quality of their processed gases including. The validation of a compressed air system in the pharmaceutical industry is typically performed according to. Usp Requirements For Compressed Air.
From nationalsafety.wordpress.com
Top 10 Compressed Air Safety Guidelines (Infographic) Nationalsafety Usp Requirements For Compressed Air The usp (the us pharmacopoeia) handle the testing of water or oil content quite easily: National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. A reference point for the selection of. The validation of a compressed air system in the pharmaceutical industry is typically performed according to. Usp Requirements For Compressed Air.
From www.compressorpros.com
How to Size a Compressor Usp Requirements For Compressed Air For compressed air applications outside the clean room, the quality requirements are less clearly defined. The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical. Usp Requirements For Compressed Air.
From www.epstec.com
Compressed air requirements for EPS thermoforming machine EPSTEC Usp Requirements For Compressed Air Using the guidance of the us fda and ispe gpg, pharmaceutical manufacturers can properly evaluate the quality of their processed gases including. National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. For compressed air applications outside the clean room, the quality requirements are less clearly defined. The. Usp Requirements For Compressed Air.
From studylib.net
Compressed Air Glossary Usp Requirements For Compressed Air Compressed air validation including tests like determination of moisture content, presence of oil content and microbial evaluation. The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. For compressed air applications outside the clean room, the quality requirements are less clearly defined. Labeling— where. Usp Requirements For Compressed Air.
From blog.gotopac.com
USP 800 Standards Upgrading a USP 797 Cleanroom for Hazardous Drugs Usp Requirements For Compressed Air For compressed air applications outside the clean room, the quality requirements are less clearly defined. Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. The usp (the us pharmacopoeia) handle the testing of water or oil content quite easily: Compressed air validation including tests. Usp Requirements For Compressed Air.
From www.youtube.com
Compressed air safety OSHA compliance YouTube Usp Requirements For Compressed Air For compressed air applications outside the clean room, the quality requirements are less clearly defined. The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical. Usp Requirements For Compressed Air.
From dailymed.nlm.nih.gov
AIR USP COMPRESSED Usp Requirements For Compressed Air Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. A reference point for the selection of. Compressed air validation including tests like determination of moisture content, presence of oil content and microbial evaluation. For compressed air applications outside the clean room, the quality requirements. Usp Requirements For Compressed Air.
From www.propharmacleanrooms.com
HVAC Design for USP 797 & 800 Cleanrooms — ProPharma Cleanrooms Usp Requirements For Compressed Air Labeling— where it is piped directly from the collecting tank to the point of use, label each outlet “medical air.” water and oil— support 1. For compressed air applications outside the clean room, the quality requirements are less clearly defined. A reference point for the selection of. Compressed air validation including tests like determination of moisture content, presence of oil. Usp Requirements For Compressed Air.
From www.slideserve.com
PPT Summary of USP 797 for Compounding Sterile Preparations Usp Requirements For Compressed Air National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. For compressed air applications outside the clean room, the quality requirements are less clearly defined. The usp (the us pharmacopoeia) handle the testing of water or oil content quite easily: Labeling— where it is piped directly from the. Usp Requirements For Compressed Air.
From www.airbestpractices.com
Using ISO 85731 to Test Compressed Air Clearing the Confusion Usp Requirements For Compressed Air The validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and guidelines provided by regulatory authorities such as. National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. Compressed air validation including tests like determination of moisture content, presence of. Usp Requirements For Compressed Air.