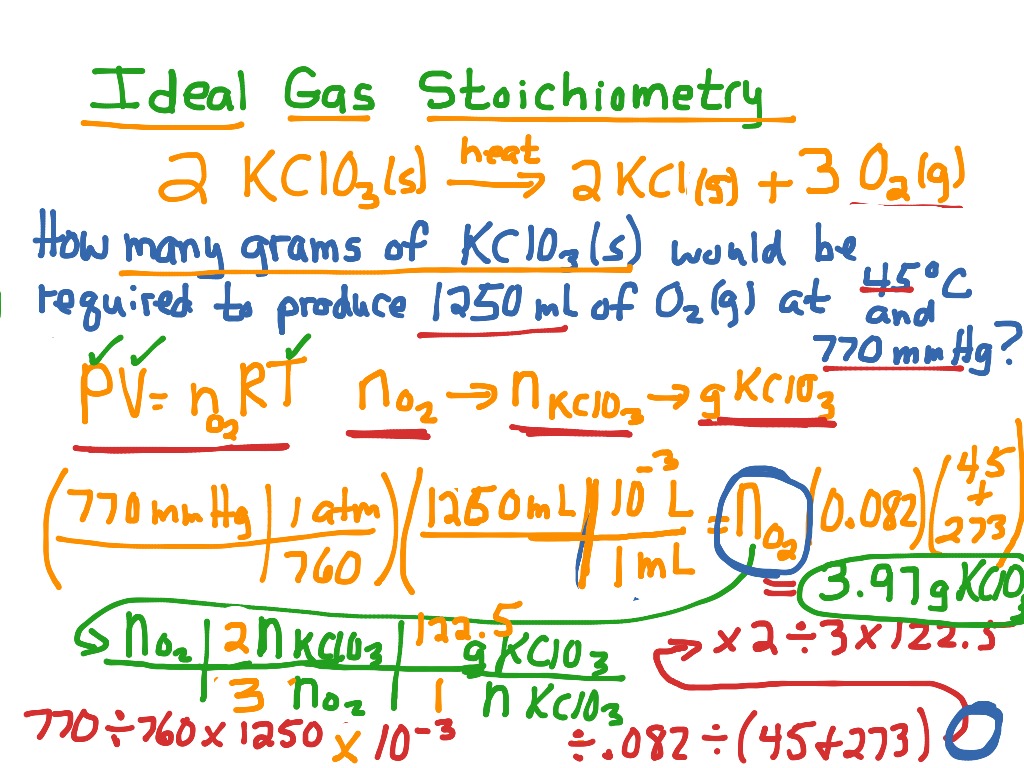
Gas Stoichiometry Quizlet . gas stoichiometry is the quantitative relationship between reactants and products in a gas reaction. Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. the ideal gas law relates the four independent physical properties of a gas at any time. study with quizlet and memorize flashcards containing terms like volume, temperature, molecules present, pressure, when gas. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. study with quizlet and memorize flashcards containing terms like pressure, kinetic molecular theory, stp (standard. to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases.
from www.showme.com
study with quizlet and memorize flashcards containing terms like pressure, kinetic molecular theory, stp (standard. study with quizlet and memorize flashcards containing terms like volume, temperature, molecules present, pressure, when gas. to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. gas stoichiometry is the quantitative relationship between reactants and products in a gas reaction. the ideal gas law relates the four independent physical properties of a gas at any time.
ShowMe Gas Stoichiometry
Gas Stoichiometry Quizlet study with quizlet and memorize flashcards containing terms like pressure, kinetic molecular theory, stp (standard. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. study with quizlet and memorize flashcards containing terms like pressure, kinetic molecular theory, stp (standard. study with quizlet and memorize flashcards containing terms like volume, temperature, molecules present, pressure, when gas. gas stoichiometry is the quantitative relationship between reactants and products in a gas reaction. study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. the ideal gas law relates the four independent physical properties of a gas at any time.
From www.sliderbase.com
Stoichiometry, Gas Stoichiometry Presentation Chemistry Gas Stoichiometry Quizlet gas stoichiometry is the quantitative relationship between reactants and products in a gas reaction. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. study with quizlet and memorize flashcards containing terms like pressure, kinetic molecular theory, stp (standard. to account for these conditions, we use. Gas Stoichiometry Quizlet.
From www.sliderbase.com
Stoichiometry, Gas Stoichiometry Presentation Chemistry Gas Stoichiometry Quizlet Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. study with quizlet and memorize flashcards containing terms like pressure, kinetic molecular theory, stp (standard. study with quizlet and memorize flashcards containing terms like volume,. Gas Stoichiometry Quizlet.
From www.youtube.com
Gas Law Stoichiometry not at STP YouTube Gas Stoichiometry Quizlet if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. the ideal gas law relates the four independent physical properties of a gas at any time. study with quizlet. Gas Stoichiometry Quizlet.
From www.slideserve.com
PPT Gas Stoichiometry PowerPoint Presentation, free download ID4199794 Gas Stoichiometry Quizlet if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. study with quizlet and memorize flashcards containing terms like volume, temperature, molecules present, pressure, when gas. study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. The ideal gas law. Gas Stoichiometry Quizlet.
From www.sliderbase.com
Stoichiometry, Gas Stoichiometry Presentation Chemistry Gas Stoichiometry Quizlet Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. gas stoichiometry is the quantitative relationship between reactants and products in a gas reaction. study with quizlet and memorize flashcards containing terms like pressure, kinetic. Gas Stoichiometry Quizlet.
From www.slideserve.com
PPT Gas Stoichiometry At STP PowerPoint Presentation, free download Gas Stoichiometry Quizlet study with quizlet and memorize flashcards containing terms like volume, temperature, molecules present, pressure, when gas. gas stoichiometry is the quantitative relationship between reactants and products in a gas reaction. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. study with quizlet and memorize flashcards containing terms like magnesium reacts with. Gas Stoichiometry Quizlet.
From slideplayer.com
IV. Gas Stoichiometry at NonSTP Conditions (p ) ppt download Gas Stoichiometry Quizlet to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. study. Gas Stoichiometry Quizlet.
From lessonfullmattie.z21.web.core.windows.net
Gas Stoichiometry Practice Worksheet Gas Stoichiometry Quizlet gas stoichiometry is the quantitative relationship between reactants and products in a gas reaction. study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. study with quizlet and memorize. Gas Stoichiometry Quizlet.
From www.slideserve.com
PPT II. Ideal Gas Law PowerPoint Presentation, free download ID3461705 Gas Stoichiometry Quizlet Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. if. Gas Stoichiometry Quizlet.
From www.youtube.com
Gas Stoichiometry Problems YouTube Gas Stoichiometry Quizlet to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. the ideal gas law relates the four independent physical properties of a gas at any time. The ideal gas law can be. Gas Stoichiometry Quizlet.
From slideplayer.com
IV. Gas Stoichiometry at NonSTP Conditions (p ) ppt download Gas Stoichiometry Quizlet to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. the ideal gas law relates the four independent physical properties of a gas at any time. study with quizlet and memorize. Gas Stoichiometry Quizlet.
From www.youtube.com
Gas Stoichiometry Examples 1 & 2 YouTube Gas Stoichiometry Quizlet to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. the ideal gas law. Gas Stoichiometry Quizlet.
From slideplayer.com
IV. Gas Stoichiometry at NonSTP Conditions (p ) ppt download Gas Stoichiometry Quizlet the ideal gas law relates the four independent physical properties of a gas at any time. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. gas stoichiometry is. Gas Stoichiometry Quizlet.
From quizlet.com
AP Chemistry Stoichiometry Terms Diagram Quizlet Gas Stoichiometry Quizlet study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. study with quizlet and. Gas Stoichiometry Quizlet.
From www.slideserve.com
PPT Gas Stoichiometry PowerPoint Presentation, free download ID4199794 Gas Stoichiometry Quizlet gas stoichiometry is the quantitative relationship between reactants and products in a gas reaction. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. study with quizlet and memorize flashcards containing terms like pressure, kinetic. Gas Stoichiometry Quizlet.
From www.showme.com
ShowMe Gas Stoichiometry Gas Stoichiometry Quizlet study with quizlet and memorize flashcards containing terms like pressure, kinetic molecular theory, stp (standard. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. gas stoichiometry is the quantitative relationship between. Gas Stoichiometry Quizlet.
From www.sliderbase.com
Stoichiometry, Gas Stoichiometry Presentation Chemistry Gas Stoichiometry Quizlet The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. the ideal gas law relates the four independent physical properties of a gas at any time. to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. if we know the volume, pressure, and temperature. Gas Stoichiometry Quizlet.
From www.youtube.com
Gas Stoichiometry STP and NonSTP Examples, Practice Problems Gas Stoichiometry Quizlet study with quizlet and memorize flashcards containing terms like volume, temperature, molecules present, pressure, when gas. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. study with quizlet. Gas Stoichiometry Quizlet.
From www.studocu.com
Gas Stoichiometry InClass Notes Gas Stoichiometry, Partial Pressure Gas Stoichiometry Quizlet Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. The ideal gas law can. Gas Stoichiometry Quizlet.
From www.studocu.com
170 Gas Stoichiometry Example Gas Stoichiometry Example Quicklime Gas Stoichiometry Quizlet to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. the ideal gas law relates the four. Gas Stoichiometry Quizlet.
From slideplayer.com
IV. Gas Stoichiometry at NonSTP Conditions (p ) ppt download Gas Stoichiometry Quizlet gas stoichiometry is the quantitative relationship between reactants and products in a gas reaction. study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. study with quizlet and memorize flashcards containing terms like volume, temperature, molecules present, pressure, when gas. if we know the volume, pressure, and temperature of. Gas Stoichiometry Quizlet.
From www.youtube.com
CHEMISTRY 101 Stoichiometry with gases YouTube Gas Stoichiometry Quizlet study with quizlet and memorize flashcards containing terms like pressure, kinetic molecular theory, stp (standard. study with quizlet and memorize flashcards containing terms like volume, temperature, molecules present, pressure, when gas. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. study with quizlet and memorize flashcards containing terms like magnesium reacts. Gas Stoichiometry Quizlet.
From www.studypool.com
SOLUTION 110 ws gas stoichiometry key Studypool Gas Stoichiometry Quizlet the ideal gas law relates the four independent physical properties of a gas at any time. to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. The ideal gas law can be. Gas Stoichiometry Quizlet.
From www.chemistrylearner.com
Free Printable Gas Stoichiometry Worksheets Gas Stoichiometry Quizlet study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. study with. Gas Stoichiometry Quizlet.
From www.scribd.com
Gas Stoichiometry Practice SOLUTIONS PDF Gas Stoichiometry Quizlet if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. the ideal gas law relates the four independent physical properties of a gas at any time. study with quizlet and memorize flashcards containing terms like volume, temperature, molecules present, pressure, when gas. to account for these. Gas Stoichiometry Quizlet.
From www.youtube.com
Gas Stoichiometry VolumeMass at STP YouTube Gas Stoichiometry Quizlet The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. study with quizlet and memorize flashcards containing terms like volume, temperature, molecules present, pressure, when gas. to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. if we know the volume, pressure, and temperature. Gas Stoichiometry Quizlet.
From www.slideserve.com
PPT Gas Stoichiometry PowerPoint Presentation, free download ID4199794 Gas Stoichiometry Quizlet study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. gas stoichiometry is the quantitative relationship between reactants and products in a gas reaction. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. the ideal gas law relates. Gas Stoichiometry Quizlet.
From www.slideserve.com
PPT Gas Stoichiometry PowerPoint Presentation, free download ID2956253 Gas Stoichiometry Quizlet to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. study with quizlet and memorize flashcards containing terms like volume, temperature, molecules present, pressure, when gas. study with. Gas Stoichiometry Quizlet.
From www.youtube.com
Gas Law Stoichiometry YouTube Gas Stoichiometry Quizlet the ideal gas law relates the four independent physical properties of a gas at any time. study with quizlet and memorize flashcards containing terms like pressure, kinetic molecular theory, stp (standard. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. study with quizlet and memorize flashcards containing terms like magnesium reacts. Gas Stoichiometry Quizlet.
From quizizz.com
50+ stoichiometry worksheets on Quizizz Free & Printable Gas Stoichiometry Quizlet study with quizlet and memorize flashcards containing terms like pressure, kinetic molecular theory, stp (standard. study with quizlet and memorize flashcards containing terms like magnesium reacts with hydrochloric acid (hcl) as follows. the ideal gas law relates the four independent physical properties of a gas at any time. study with quizlet and memorize flashcards containing terms. Gas Stoichiometry Quizlet.
From rumble.com
Stoichiometry, ideal gas law, example Chemistry Gas Stoichiometry Quizlet study with quizlet and memorize flashcards containing terms like volume, temperature, molecules present, pressure, when gas. to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. study with quizlet and memorize flashcards containing terms like pressure, kinetic molecular theory, stp (standard. if we know the volume, pressure, and. Gas Stoichiometry Quizlet.
From www.slideserve.com
PPT Gas Stoichiometry PowerPoint Presentation, free download ID4199794 Gas Stoichiometry Quizlet the ideal gas law relates the four independent physical properties of a gas at any time. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. gas stoichiometry is the quantitative relationship. Gas Stoichiometry Quizlet.
From www.slideserve.com
PPT Gas Stoichiometry PowerPoint Presentation, free download ID2428278 Gas Stoichiometry Quizlet Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. the ideal gas law relates the four independent physical properties of a gas at any time. gas stoichiometry is the quantitative relationship between reactants and products in a gas reaction. if we know the volume, pressure, and temperature of. Gas Stoichiometry Quizlet.
From teachsimple.com
Practice Gas Stoichiometry by Teach Simple Gas Stoichiometry Quizlet study with quizlet and memorize flashcards containing terms like pressure, kinetic molecular theory, stp (standard. Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. to account for these conditions, we use the ideal gas equation pv=nrt where p is the pressure measured. gas stoichiometry is the quantitative relationship. Gas Stoichiometry Quizlet.
From www.youtube.com
Gas Law Stoichiometry Worksheet YouTube Gas Stoichiometry Quizlet the ideal gas law relates the four independent physical properties of a gas at any time. if we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate. study with quizlet and memorize flashcards containing terms like volume, temperature, molecules present, pressure, when gas. Standard temperature and pressure (stp). Gas Stoichiometry Quizlet.