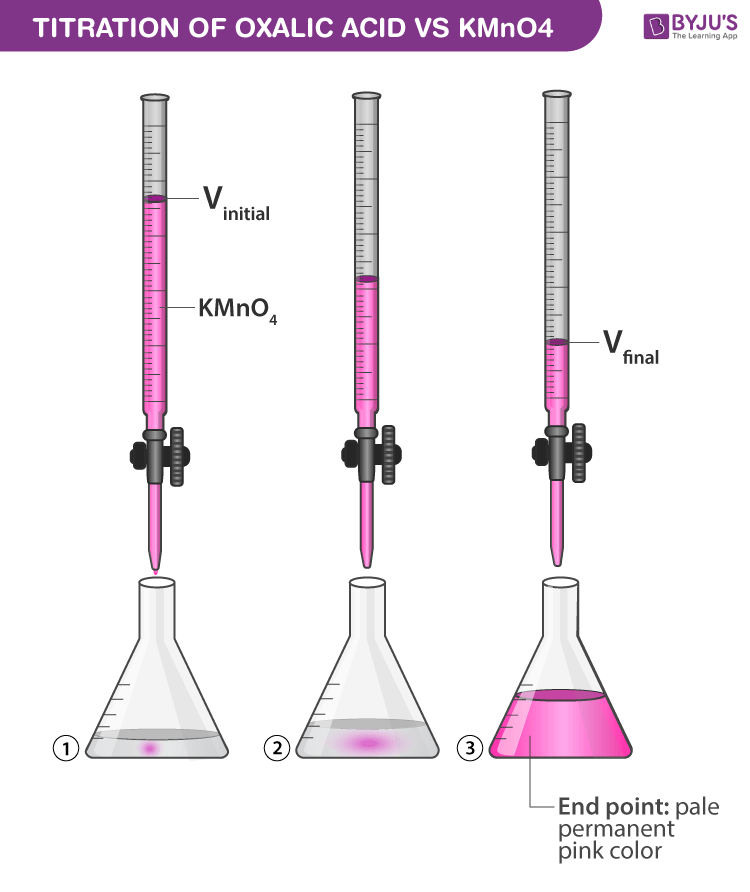
Redox Titration Indicators . Redox titration is a laboratory method of determining the concentration of a given analyte by causing a. It may involve the use. what is redox titration? The oxidized and reduced forms of some titrants, such as. a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant. a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. three types of indicators are used to signal a redox titration’s end point. redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction.
from dxopjnnhs.blob.core.windows.net
what is redox titration? a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant. a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a. common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. three types of indicators are used to signal a redox titration’s end point. It may involve the use. The oxidized and reduced forms of some titrants, such as.
A Level Chemistry Core Practical 11 Redox Titration at Abby Moseley blog
Redox Titration Indicators three types of indicators are used to signal a redox titration’s end point. a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant. It may involve the use. a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. The oxidized and reduced forms of some titrants, such as. three types of indicators are used to signal a redox titration’s end point. redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction. what is redox titration? common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Redox Titration Indicators Redox titration is a laboratory method of determining the concentration of a given analyte by causing a. The oxidized and reduced forms of some titrants, such as. redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction. It may involve the use. a redox indicator is a substance used in. Redox Titration Indicators.
From www.slideserve.com
PPT 4.1 Redox Titrations By Dr. P. B. Thakur PowerPoint Presentation ID9522386 Redox Titration Indicators a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a. The oxidized and reduced forms of some titrants, such as. three types of indicators are used to signal a redox titration’s end. Redox Titration Indicators.
From www.youtube.com
Redox Titration Oxidation Reduction Redox Indicators Pharmaceutical Analysis B Pharma Redox Titration Indicators It may involve the use. three types of indicators are used to signal a redox titration’s end point. redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction. common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. The oxidized and reduced. Redox Titration Indicators.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID1154145 Redox Titration Indicators It may involve the use. a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant. common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. three types of indicators are used to signal a redox titration’s end point. Redox titration is. Redox Titration Indicators.
From pngset.com
Redox Titration Using Indicator, Technology, Rocket, Transportation, Droplet Transparent Png Redox Titration Indicators three types of indicators are used to signal a redox titration’s end point. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a. The oxidized and reduced forms of some titrants, such as. what is redox titration? a redox titration [1] is a type of titration based on a redox. Redox Titration Indicators.
From www.studypool.com
SOLUTION Chem2001 redox titrations and indicators Studypool Redox Titration Indicators The oxidized and reduced forms of some titrants, such as. what is redox titration? common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. three types of indicators are used to signal a redox titration’s end point. redox titration is a titration in which the reaction between the analyte. Redox Titration Indicators.
From www.studypool.com
SOLUTION Chem2001 redox titrations and indicators Studypool Redox Titration Indicators Redox titration is a laboratory method of determining the concentration of a given analyte by causing a. It may involve the use. a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. The oxidized and reduced forms of some titrants, such as. redox titration is a titration in which. Redox Titration Indicators.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 Redox Titration Indicators The oxidized and reduced forms of some titrants, such as. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a. a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant. common indicators utilized in redox titrations include organic dyes and compounds sensitive. Redox Titration Indicators.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Redox Titration Indicators redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction. It may involve the use. common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. a redox titration [1] is a type of titration based on a redox reaction between the analyte. Redox Titration Indicators.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID3080571 Redox Titration Indicators common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a. a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. The oxidized and reduced forms. Redox Titration Indicators.
From www.slideserve.com
PPT 4.1 Redox Titrations By Dr. P. B. Thakur PowerPoint Presentation ID9522386 Redox Titration Indicators a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. three types of indicators are used to signal a redox titration’s end point. It may involve the use. common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. what is. Redox Titration Indicators.
From www.youtube.com
Redox indicators (Redox TitrationPart04) Lecture67 by Sachin Rai YouTube Redox Titration Indicators a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant. The oxidized and reduced forms of some titrants, such as. common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. redox titration is a titration in which the reaction between the. Redox Titration Indicators.
From www.clipartkey.com
Redox Titration Apparatus Of Ferrous Ions By Ceric Redox Indicators For Titrations , Free Redox Titration Indicators a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant. three types of indicators are used to signal a redox titration’s end point. The oxidized and reduced forms of some titrants, such as. common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in. Redox Titration Indicators.
From school.careers360.com
redox titration Overview, Structure, Properties & Uses Redox Titration Indicators what is redox titration? Redox titration is a laboratory method of determining the concentration of a given analyte by causing a. It may involve the use. a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant. a redox indicator is a substance used in titrations to signal when. Redox Titration Indicators.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID1154145 Redox Titration Indicators redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction. three types of indicators are used to signal a redox titration’s end point. a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. Redox titration is a laboratory method of. Redox Titration Indicators.
From scienceinfo.com
Redox indicators Redox Titration Indicators what is redox titration? common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. It may involve the use. three types of indicators are used to signal a redox titration’s end point. The oxidized and reduced forms of some titrants, such as. redox titration is a titration in which. Redox Titration Indicators.
From www.youtube.com
Redox titration using Internal indicator YouTube Redox Titration Indicators a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant. what is redox titration? three types of indicators are used to signal a redox titration’s end point. a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. . Redox Titration Indicators.
From www.youtube.com
Self Indicators Redox Titration Analytical Chemistry Internal Indicators YouTube Redox Titration Indicators a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. three types of indicators are used to signal a redox titration’s end point. The oxidized and reduced forms of some titrants, such as. It may involve the use. a redox titration [1] is a type of titration based. Redox Titration Indicators.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Redox Titration Indicators three types of indicators are used to signal a redox titration’s end point. a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. what is redox titration? a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant. The. Redox Titration Indicators.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID1154145 Redox Titration Indicators common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. It may involve the use. redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction. The oxidized and reduced forms of some titrants, such as. three types of indicators are used to. Redox Titration Indicators.
From www.youtube.com
Indicators in redox titration redox titration indicators redox titration analysis Redox Titration Indicators what is redox titration? three types of indicators are used to signal a redox titration’s end point. a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction. The oxidized. Redox Titration Indicators.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Redox Titration Indicators a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. three types of indicators are used to signal a redox titration’s end point. a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant. The oxidized and reduced forms of. Redox Titration Indicators.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID3080571 Redox Titration Indicators common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. The oxidized and reduced forms of some titrants, such as. a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. a redox titration [1] is a type of titration based on. Redox Titration Indicators.
From www.scribd.com
Redox Indicator There Are Two Common Types of Redox Indicators PDF Redox Titration Redox Titration Indicators what is redox titration? Redox titration is a laboratory method of determining the concentration of a given analyte by causing a. redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction. The oxidized and reduced forms of some titrants, such as. three types of indicators are used to signal. Redox Titration Indicators.
From dxopjnnhs.blob.core.windows.net
A Level Chemistry Core Practical 11 Redox Titration at Abby Moseley blog Redox Titration Indicators common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. It may involve the use. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a. a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant.. Redox Titration Indicators.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Redox Titration Indicators a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a. common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. redox titration is a. Redox Titration Indicators.
From slidetodoc.com
Redox Titrations Introduction 1 Redox Titration Based on Redox Titration Indicators redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction. It may involve the use. a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. Redox titration is a laboratory method of determining the concentration of a given analyte by causing. Redox Titration Indicators.
From slidetodoc.com
Chapter 15 Redox Titrations 15 1 The Shape Redox Titration Indicators what is redox titration? a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant. common indicators utilized in redox titrations include organic dyes and compounds sensitive to changes in oxidation states. three types of indicators are used to signal a redox titration’s end point. The oxidized and. Redox Titration Indicators.
From chemistnotes.com
Redox Titration Definition, Requirements, Indicators, and 5 Reliable Applications Chemistry Notes Redox Titration Indicators redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction. what is redox titration? a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. The oxidized and reduced forms of some titrants, such as. It may involve the use. . Redox Titration Indicators.
From www.freeimages.com
Free redox titration using indicator illustrations Download free stock images FreeImages Redox Titration Indicators redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction. a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant. a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. . Redox Titration Indicators.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Redox Titration Indicators The oxidized and reduced forms of some titrants, such as. three types of indicators are used to signal a redox titration’s end point. a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. a redox titration [1] is a type of titration based on a redox reaction between. Redox Titration Indicators.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID1154145 Redox Titration Indicators a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. The oxidized and reduced forms of some titrants, such as. three types of indicators are used to signal a redox titration’s end point. a redox titration [1] is a type of titration based on a redox reaction between. Redox Titration Indicators.
From www.youtube.com
Redox Titration II KMnO4 vs Oxalic acid II Self indicator YouTube Redox Titration Indicators redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction. what is redox titration? a redox titration [1] is a type of titration based on a redox reaction between the analyte and titrant. three types of indicators are used to signal a redox titration’s end point. The oxidized. Redox Titration Indicators.
From www.slideserve.com
PPT 4.1 Redox Titrations By Dr. P. B. Thakur PowerPoint Presentation ID9522386 Redox Titration Indicators It may involve the use. redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction. a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. The oxidized and reduced forms of some titrants, such as. a redox titration [1] is. Redox Titration Indicators.
From chemistnotes.com
Redox Titration Definition, Requirements, Indicators, and 5 Reliable Applications Chemistry Notes Redox Titration Indicators redox titration is a titration in which the reaction between the analyte and titrant is an oxidation/reduction reaction. a redox indicator is a substance used in titrations to signal when the solution's potential has reached equivalence by. The oxidized and reduced forms of some titrants, such as. three types of indicators are used to signal a redox. Redox Titration Indicators.