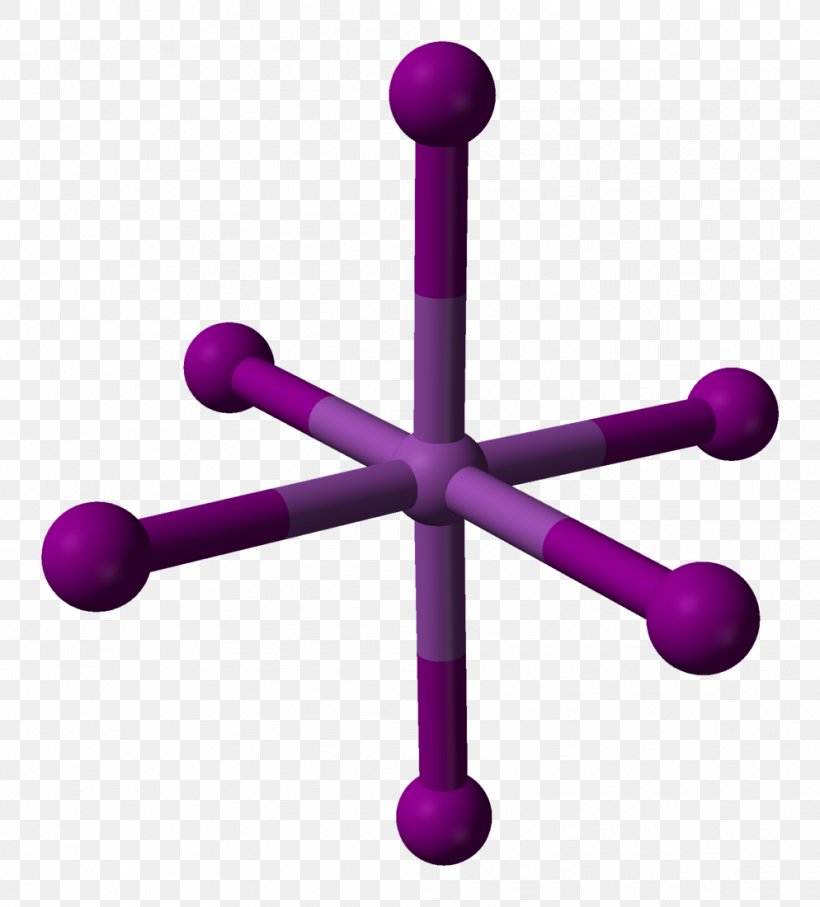
Magnesium Iodide Is . Magnesium iodide is an inorganic compound with the chemical formula mg i 2. I 2 mg molecular weight: Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. M g2+ +2i − → m gi 2. Magnesium metal forms a doubly charged cation. It forms various hydrates mgi2·xh2o. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Iodide forms a singly charged anion.
from favpng.com
I 2 mg molecular weight: Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Magnesium metal forms a doubly charged cation. M g2+ +2i − → m gi 2. Iodide forms a singly charged anion. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. It forms various hydrates mgi2·xh2o.
Bismuth(III) Iodide Magnesium Iodide Bismuth Chloride Crystal Structure
Magnesium Iodide Is Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. I 2 mg molecular weight: Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Iodide forms a singly charged anion. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. It forms various hydrates mgi2·xh2o. M g2+ +2i − → m gi 2. Magnesium metal forms a doubly charged cation.
From brainly.in
what is the symbol of magnesium iodide Brainly.in Magnesium Iodide Is M g2+ +2i − → m gi 2. Magnesium metal forms a doubly charged cation. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. It forms various hydrates mgi2·xh2o. I 2 mg molecular weight: Magnesium iodide is a highly stable compound under normal conditions, but it. Magnesium Iodide Is.
From www.doubtnut.com
[Odia] When nbutyl magnesium iodide is treated with water the product Magnesium Iodide Is Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. I 2 mg molecular weight: Magnesium metal forms a doubly charged cation. It forms various hydrates mgi2·xh2o. M g2+ +2i − → m gi 2. Iodide forms a singly charged anion. Magnesium iodide is an inorganic compound with the chemical formula mg i. Magnesium Iodide Is.
From procure-net.com
Pharmaceutical Grade Magnesium Iodide Purity & Versatility Rolled Magnesium Iodide Is Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Iodide forms a singly charged anion. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Magnesium metal forms a doubly charged cation. Magnesium iodide is a highly stable compound. Magnesium Iodide Is.
From www.indiamart.com
White Magnesium Iodide Anhydrous, for Laboratory at best price in Vadodara Magnesium Iodide Is Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. M g2+ +2i − → m gi 2. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Iodide forms a singly charged anion. Magnesium iodide is an inorganic compound with the chemical formula mg i. Magnesium Iodide Is.
From www.nanochemazone.com
Magnesium Iodide Powder Low Price 1 Nanochemazone Magnesium Iodide Is Iodide forms a singly charged anion. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. It forms various hydrates mgi2·xh2o. M g2+ +2i − → m gi 2. In the magnesium iodide compound, magnesium loses two electrons to become. Magnesium Iodide Is.
From favpng.com
Magnesium Iodide Chemical Compound Hydrate, PNG, 1033x1100px, Magnesium Magnesium Iodide Is I 2 mg molecular weight: M g2+ +2i − → m gi 2. Magnesium metal forms a doubly charged cation. It forms various hydrates mgi2·xh2o. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Magnesium iodide is a highly. Magnesium Iodide Is.
From imgbin.com
Magnesium Iodide Chemical Compound Magnesium Deficiency PNG, Clipart Magnesium Iodide Is It forms various hydrates mgi2·xh2o. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. M g2+ +2i − → m gi 2. Magnesium iodide is a highly stable compound. Magnesium Iodide Is.
From byjus.com
Acetone is obtained by the hydrolysis of the addition product of methyl Magnesium Iodide Is Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. I 2 mg molecular weight: Iodide forms a singly charged anion. Magnesium iodide is an ionic compound formed by the. Magnesium Iodide Is.
From www.indiamart.com
Magnesium Iodide at Rs 59.46 Kandivali West Mumbai ID 24673998630 Magnesium Iodide Is Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Magnesium metal forms a doubly charged cation. It forms various hydrates mgi2·xh2o. M g2+ +2i − → m gi 2. Iodide forms a singly charged anion. In the magnesium iodide. Magnesium Iodide Is.
From www.fishersci.co.uk
Magnesium iodide, ultra dry, 99.996 (metals basis), Thermo Scientific Magnesium Iodide Is Magnesium iodide is an inorganic compound with the chemical formula mg i 2. It forms various hydrates mgi2·xh2o. Magnesium metal forms a doubly charged cation. Iodide forms a singly charged anion. M g2+ +2i − → m gi 2. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. I 2 mg molecular. Magnesium Iodide Is.
From www.toppr.com
When the compound shown is treated with two equivalent of methyl Magnesium Iodide Is M g2+ +2i − → m gi 2. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻. Magnesium Iodide Is.
From exowshhvp.blob.core.windows.net
Magnesium Iodide Octahydrate at Rebecca Gonzalez blog Magnesium Iodide Is M g2+ +2i − → m gi 2. It forms various hydrates mgi2·xh2o. Iodide forms a singly charged anion. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. In the magnesium iodide compound, magnesium loses two electrons to become. Magnesium Iodide Is.
From www.youtube.com
Preparation & Properties of Magnesium iodide YouTube Magnesium Iodide Is It forms various hydrates mgi2·xh2o. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Magnesium metal forms a doubly charged cation. M g2+ +2i − → m gi 2. In the magnesium iodide compound, magnesium loses two electrons to. Magnesium Iodide Is.
From www.mahaautomation.com
METHYL MAGNESIUM IODIDE 3.0M IN DIETHYL ETHER Magnesium Iodide Is Magnesium metal forms a doubly charged cation. It forms various hydrates mgi2·xh2o. M g2+ +2i − → m gi 2. Iodide forms a singly charged anion. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. I 2 mg molecular weight: Magnesium iodide is a highly stable. Magnesium Iodide Is.
From www.indiamart.com
Magnesium Iodide Anhydrous at best price in Vadodara by Axiom Chemicals Magnesium Iodide Is Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Magnesium iodide is an ionic compound formed. Magnesium Iodide Is.
From brainly.com
The diagram shows how magnesium and iodine atoms form magnesium iodide Magnesium Iodide Is Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. M g2+ +2i − → m gi 2. Magnesium metal forms a doubly charged cation. It forms various hydrates mgi2·xh2o. Iodide forms a singly charged anion. Magnesium iodide is a. Magnesium Iodide Is.
From www.dreamstime.com
MgI2 Magnesium Iodide CAS 10377589 Chemical Substance in White Magnesium Iodide Is Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Iodide forms a singly charged anion. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Magnesium. Magnesium Iodide Is.
From molekula.com
Purchase Magnesium iodide anhydrous [10377589] online • Catalog Magnesium Iodide Is Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. It forms various hydrates mgi2·xh2o. Iodide forms a singly charged anion. Magnesium metal forms a doubly charged cation. Magnesium iodide. Magnesium Iodide Is.
From www.doubtnut.com
Ethyl magnesium iodide reacts with propylamine to give Magnesium Iodide Is Magnesium iodide is an inorganic compound with the chemical formula mg i 2. It forms various hydrates mgi2·xh2o. Magnesium metal forms a doubly charged cation. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. M. Magnesium Iodide Is.
From www.vedantu.com
The reaction of methyl magnesium iodide with acetone followed by Magnesium Iodide Is Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. Iodide forms a singly charged anion. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. It forms various hydrates mgi2·xh2o. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron. Magnesium Iodide Is.
From wiringfixbugatti.z19.web.core.windows.net
Magnesium And Iodide Electron Dot Diagram Magnesium Iodide Is Magnesium iodide is an inorganic compound with the chemical formula mg i 2. It forms various hydrates mgi2·xh2o. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. I 2. Magnesium Iodide Is.
From www.doubtnut.com
Methyl magnesium iodide is converted to ethyl magnesium iodide Magnesium Iodide Is It forms various hydrates mgi2·xh2o. Iodide forms a singly charged anion. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. I 2. Magnesium Iodide Is.
From www.pw.live
Magnesium Iodide Formula, Structure And Properties Magnesium Iodide Is Iodide forms a singly charged anion. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. Magnesium metal forms a doubly charged cation. I 2 mg molecular weight: It forms various hydrates mgi2·xh2o. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to. Magnesium Iodide Is.
From favpng.com
Bismuth(III) Iodide Magnesium Iodide Bismuth Chloride Crystal Structure Magnesium Iodide Is Magnesium iodide is an inorganic compound with the chemical formula mg i 2. I 2 mg molecular weight: Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. It forms various hydrates mgi2·xh2o. M g2+ +2i − → m gi 2. Magnesium metal forms a doubly charged cation. In the magnesium iodide compound,. Magnesium Iodide Is.
From guides.byjusweb.com
Magnesium Iodide Formula Definition, Concepts and Examples Magnesium Iodide Is It forms various hydrates mgi2·xh2o. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. M g2+ +2i − → m gi 2. Iodide forms a singly charged anion. Magnesium. Magnesium Iodide Is.
From www.youtube.com
How to Balance Mg + I2 = MgI2 (Magnesium + Iodine gas) YouTube Magnesium Iodide Is Iodide forms a singly charged anion. Magnesium metal forms a doubly charged cation. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. It forms various hydrates mgi2·xh2o. I 2 mg molecular weight: M g2+ +2i − → m gi 2. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine. Magnesium Iodide Is.
From www.numerade.com
SOLVED A 1.628 g sample of a hydrate of magnesium iodide is heated Magnesium Iodide Is Iodide forms a singly charged anion. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. M g2+ +2i − → m gi 2. Magnesium metal forms a doubly charged cation. It forms various hydrates mgi2·xh2o. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form. Magnesium Iodide Is.
From fyozmmqxl.blob.core.windows.net
Magnesium Iodide Water Equation at Ivan Koch blog Magnesium Iodide Is Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. I 2 mg molecular weight: In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. It forms various hydrates mgi2·xh2o. M g2+ +2i − → m gi 2. Magnesium iodide. Magnesium Iodide Is.
From procure-net.com
Magnesium Iodide 98 Supreme Quality Compound for Organic Synthesis Magnesium Iodide Is M g2+ +2i − → m gi 2. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. I 2 mg molecular weight: Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. It forms various hydrates mgi2·xh2o. Magnesium metal forms a doubly charged cation. Magnesium iodide is a highly. Magnesium Iodide Is.
From www.ibuychemikals.com
Buy Magnesium Iodide at ibuychemikals online platform for buying and Magnesium Iodide Is It forms various hydrates mgi2·xh2o. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. I 2. Magnesium Iodide Is.
From chemcraft.su
Magnesium iodide octahydrate, 99.8 chemcraft.su Magnesium Iodide Is It forms various hydrates mgi2·xh2o. Iodide forms a singly charged anion. Magnesium metal forms a doubly charged cation. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. M g2+ +2i − → m gi 2. I 2 mg molecular. Magnesium Iodide Is.
From www.doubtnut.com
To prepare butan2ol from methyl magnesium iodide. The compound requi Magnesium Iodide Is M g2+ +2i − → m gi 2. I 2 mg molecular weight: Magnesium iodide is an inorganic compound with the chemical formula mg i 2. It forms various hydrates mgi2·xh2o. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Iodide forms a singly charged anion.. Magnesium Iodide Is.
From www.dreamstime.com
MgI2 Magnesium Iodide stock photo. Image of poison 288820158 Magnesium Iodide Is Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. I 2 mg molecular weight: Iodide forms a singly charged anion. Magnesium metal forms a doubly charged cation. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Magnesium iodide. Magnesium Iodide Is.
From procure-net.com
HighPurity Magnesium Iodide Anhydrous Diverse Applications Magnesium Iodide Is I 2 mg molecular weight: Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Magnesium metal forms a doubly charged cation. M. Magnesium Iodide Is.
From exopjhqmk.blob.core.windows.net
Magnesium Iodide Ionic Compound Formula at Adelaide Copeland blog Magnesium Iodide Is I 2 mg molecular weight: It forms various hydrates mgi2·xh2o. Iodide forms a singly charged anion. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. M g2+ +2i − → m gi 2.. Magnesium Iodide Is.