What Is Electron Binding Energy . Electron binding energy, also called ionization potential, is the amount of energy necessary to remove an electron from an atom. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule,. Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. Electron binding energies, in electron volts, for the elements in their natural forms. The electron binding energy is the minimum energy that is required to remove an electron from an atom, as the negatively. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free. Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital.
from physicscalculations.com
Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule,. Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free. Electron binding energies, in electron volts, for the elements in their natural forms. Electron binding energy, also called ionization potential, is the amount of energy necessary to remove an electron from an atom. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. The electron binding energy is the minimum energy that is required to remove an electron from an atom, as the negatively.
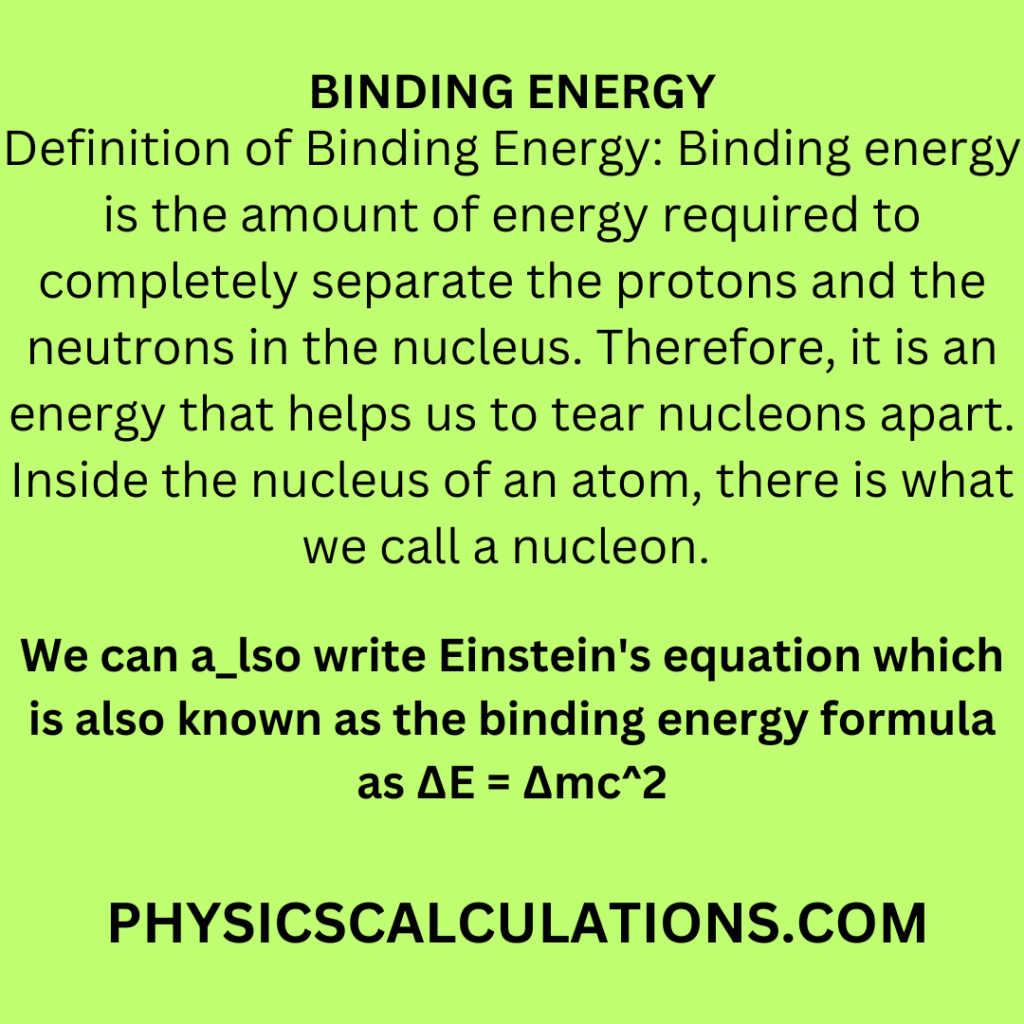
How to Calculate Binding Energy
What Is Electron Binding Energy Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule,. Electron binding energy, also called ionization potential, is the amount of energy necessary to remove an electron from an atom. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule,. Electron binding energies, in electron volts, for the elements in their natural forms. Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. The electron binding energy is the minimum energy that is required to remove an electron from an atom, as the negatively. In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons.
From www.expii.com
Nuclear Binding Energy — Definition & Overview Expii What Is Electron Binding Energy Electron binding energies, in electron volts, for the elements in their natural forms. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Electron binding energy, also called ionization potential, is the amount of energy necessary to remove an electron from an atom. In the context of atoms, binding energy refers. What Is Electron Binding Energy.
From www.researchgate.net
Molecular orbitals, electron binding energy and energy for What Is Electron Binding Energy Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule,. Electron binding energies, in electron volts, for the elements in their natural forms. The binding energy (be) of a nucleus. What Is Electron Binding Energy.
From eduinput.com
What is Binding Energy?Definition, Types, And Applications What Is Electron Binding Energy The electron binding energy is the minimum energy that is required to remove an electron from an atom, as the negatively. Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. In the context of atoms, binding energy refers to the energy required to remove an electron from an atom. What Is Electron Binding Energy.
From byjus.com
Ground state energy of an H atom is 13.6eV. The energy needed to What Is Electron Binding Energy The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Electron binding energy, also called ionization potential, is the amount of energy necessary to remove an electron from an atom. Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. In. What Is Electron Binding Energy.
From www.researchgate.net
Binding energy comparison between our model (QLM) with Cluster model What Is Electron Binding Energy In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free. Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. The binding energy (be) of a nucleus is the energy needed to separate it into. What Is Electron Binding Energy.
From studylib.net
Nuclear Binding Energy What Is Electron Binding Energy Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule,. In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and. What Is Electron Binding Energy.
From www.carlsonstockart.com
Electron Energy Levels of Atoms Carlson Stock Art What Is Electron Binding Energy Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule,. Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. The electron binding energy is the. What Is Electron Binding Energy.
From www.tessshebaylo.com
Binding Energy Equation Electron Tessshebaylo What Is Electron Binding Energy Electron binding energy, also called ionization potential, is the amount of energy necessary to remove an electron from an atom. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule,. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Electron. What Is Electron Binding Energy.
From byjus.com
If the binding energy of the electron in the ground state of hydrogen What Is Electron Binding Energy Electron binding energies, in electron volts, for the elements in their natural forms. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule,. In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free. Electron binding. What Is Electron Binding Energy.
From winter.group.shef.ac.uk
Elements Periodic Table » Periodicity » Electron binding energies (N What Is Electron Binding Energy The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Electron binding energies, in electron volts, for the elements in their natural forms. Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. Electron binding energy or ionization energy is the energy that we need to free. What Is Electron Binding Energy.
From www.researchgate.net
Binding energies (in eV) for 4p(= EI ), 4s, 3d and 3p electrons for What Is Electron Binding Energy Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. The electron binding energy is the minimum energy that is required to remove an electron from an atom, as the negatively. In the context of atoms,. What Is Electron Binding Energy.
From www.coursehero.com
[Solved] What is the binding energy of an electron in a metal whose What Is Electron Binding Energy The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free. The electron binding energy is the minimum energy that is required to remove an electron from. What Is Electron Binding Energy.
From www.slideserve.com
PPT Binding energy in atoms and nuclei PowerPoint Presentation, free What Is Electron Binding Energy Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. Electron binding energies, in electron volts, for the elements in their natural forms. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. The electron binding energy is the minimum energy that is required to remove an. What Is Electron Binding Energy.
From www.slideserve.com
PPT Physics of Radiography Chapter 4 PowerPoint Presentation, free What Is Electron Binding Energy The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free. The electron binding energy is the minimum energy that is required to remove an electron from. What Is Electron Binding Energy.
From www.youtube.com
CHEM 101 Photoelectric Effect Threshold Frequency and Binding Energy What Is Electron Binding Energy In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free. Electron binding energy, also called ionization potential, is the amount of energy necessary to remove an electron from an atom. The binding energy (be) of a nucleus is the energy needed to separate it into. What Is Electron Binding Energy.
From physicscalculations.com
How to Calculate Binding Energy What Is Electron Binding Energy In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free. The electron binding energy is the minimum energy that is required to remove an electron from an atom, as the negatively. Electron binding energies, in electron volts, for the elements in their natural forms. Element. What Is Electron Binding Energy.
From www.meritnation.com
The binding energy of electron in a metal is 250 kJ/mol The threshold What Is Electron Binding Energy Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free. Electron. What Is Electron Binding Energy.
From www.tessshebaylo.com
Exciton Binding Energy Equation Tessshebaylo What Is Electron Binding Energy Electron binding energy, also called ionization potential, is the amount of energy necessary to remove an electron from an atom. In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free. The binding energy (be) of a nucleus is the energy needed to separate it into. What Is Electron Binding Energy.
From www.tessshebaylo.com
Binding Energy Equation Physics Tessshebaylo What Is Electron Binding Energy Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Electron binding energies, in electron volts, for. What Is Electron Binding Energy.
From general.chemistrysteps.com
Photoelectric Effect Chemistry Steps What Is Electron Binding Energy Electron binding energy, also called ionization potential, is the amount of energy necessary to remove an electron from an atom. The electron binding energy is the minimum energy that is required to remove an electron from an atom, as the negatively. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons.. What Is Electron Binding Energy.
From www.youtube.com
C.7 Calculating binding energy (HL) YouTube What Is Electron Binding Energy The electron binding energy is the minimum energy that is required to remove an electron from an atom, as the negatively. Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free.. What Is Electron Binding Energy.
From byjus.com
If the binding energy of the electron in the ground state of hydrogen What Is Electron Binding Energy Electron binding energy, also called ionization potential, is the amount of energy necessary to remove an electron from an atom. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. The electron binding energy is the minimum energy that is required to remove an electron from an atom, as the negatively.. What Is Electron Binding Energy.
From general.chemistrysteps.com
Nuclear Binding Energy Chemistry Steps What Is Electron Binding Energy Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. Electron binding energy, also called ionization potential, is the amount of energy necessary to remove an electron from an atom. Electron binding energies, in electron volts, for the elements in their natural forms. Electron binding energy or ionization energy is the energy that we need to. What Is Electron Binding Energy.
From www.bartleby.com
Answered What is the binding energy (in MeV) for… bartleby What Is Electron Binding Energy Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule,. The electron binding energy is the minimum energy that is required to remove an electron from an atom, as the negatively. In the context of atoms, binding energy refers to the energy required to remove an electron from an atom. What Is Electron Binding Energy.
From www.globalsino.com
3d Elements in Periodic Table What Is Electron Binding Energy The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Electron binding energy, also called ionization potential, is the amount of energy necessary to remove an electron from an atom. Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. Electron binding energies, in electron volts, for. What Is Electron Binding Energy.
From brainly.in
draw a diagram of bohrs model of an atom showing four energy shells What Is Electron Binding Energy Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. The electron binding energy is the minimum energy that is required to remove an electron from an atom, as the negatively. Electron binding energies, in electron. What Is Electron Binding Energy.
From byjus.com
What do you mean by binding energy? What Is Electron Binding Energy Electron binding energy, also called ionization potential, is the amount of energy necessary to remove an electron from an atom. Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Electron. What Is Electron Binding Energy.
From socratic.org
How can I represent enthalpy in a potential energy diagram? Socratic What Is Electron Binding Energy Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule,. In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free. Electron binding energy or ionization energy is the energy that we need to free an. What Is Electron Binding Energy.
From www.universetoday.com
What is Binding Energy? Universe Today What Is Electron Binding Energy The electron binding energy is the minimum energy that is required to remove an electron from an atom, as the negatively. Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. Electron binding energies, in electron volts, for the elements in their natural forms. Electron binding energy, also called ionization. What Is Electron Binding Energy.
From phys.libretexts.org
10.3 Nuclear Binding Energy Physics LibreTexts What Is Electron Binding Energy Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule,. In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free. The electron binding energy is the minimum energy that is required to remove an electron. What Is Electron Binding Energy.
From www.researchgate.net
Electron binding energies (in keV) for the rare gas atoms [22 What Is Electron Binding Energy In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free. Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. Electron. What Is Electron Binding Energy.
From www.sciencemadness.org
Sciencemadness Discussion Board Ionization energy. Powered by XMB 1 What Is Electron Binding Energy Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule,. Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. The binding energy (be) of a. What Is Electron Binding Energy.
From www.chegg.com
Solved What is the binding energy (in eV ) of the electron What Is Electron Binding Energy Electron binding energy or ionization energy is the energy that we need to free an electron from its atomic orbital. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. The electron binding energy is the minimum energy that is required to remove an electron from an atom, as the negatively.. What Is Electron Binding Energy.
From www.slideserve.com
PPT Part 2 PowerPoint Presentation, free download ID3597841 What Is Electron Binding Energy The electron binding energy is the minimum energy that is required to remove an electron from an atom, as the negatively. In the context of atoms, binding energy refers to the energy required to remove an electron from an atom and release it as a free. Electron binding energies, in electron volts, for the elements in their natural forms. The. What Is Electron Binding Energy.
From slidesharenow.blogspot.com
What Is Binding Energy slideshare What Is Electron Binding Energy Element k 1s l1 2s l2 2p1/2 l3 2p3/2 m1 3s m2 3p1/2 m3. Electron binding energy, also called ionization potential, is the amount of energy necessary to remove an electron from an atom. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule,. The electron binding energy is the. What Is Electron Binding Energy.