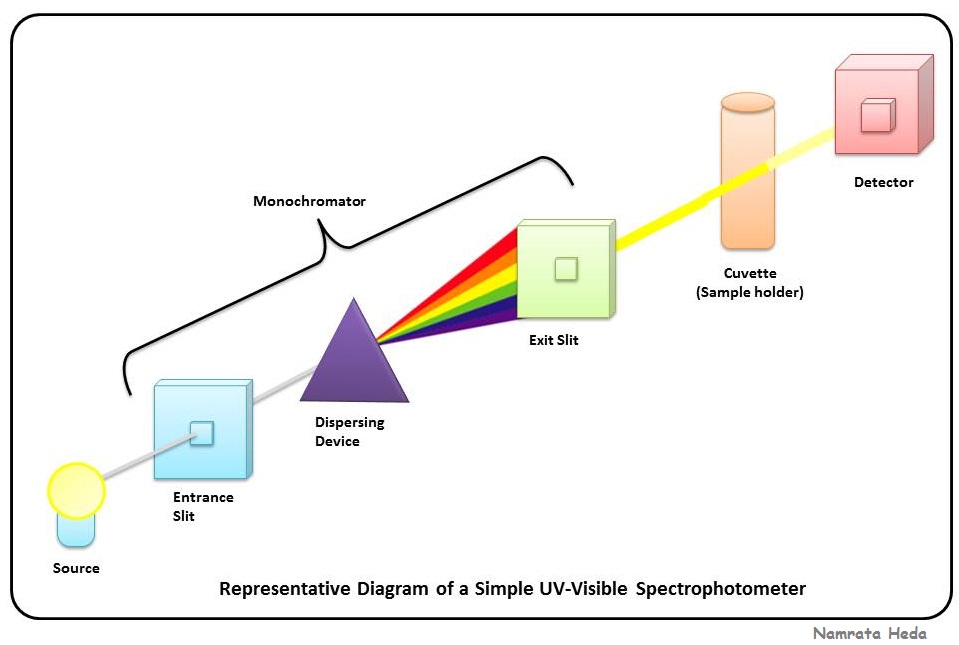
What Does High Absorbance Mean In Spectrophotometry . Transmittance is the quantity of light that passes through a. The second step of the process is to generate a standard curve. The standard curve is generated by. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. Higher absorbance indicates stronger absorption. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. For example, techniques like the bca assay allow scientists to estimate the. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max.
from namrataheda.blogspot.com
The second step of the process is to generate a standard curve. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. The standard curve is generated by. Transmittance is the quantity of light that passes through a. For example, techniques like the bca assay allow scientists to estimate the. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Higher absorbance indicates stronger absorption.
B for Biology Spectrophotometry UVVisible Spectrophotometry
What Does High Absorbance Mean In Spectrophotometry Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. The standard curve is generated by. Higher absorbance indicates stronger absorption. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Transmittance is the quantity of light that passes through a. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. The second step of the process is to generate a standard curve. For example, techniques like the bca assay allow scientists to estimate the.
From www.researchgate.net
a UVVis optical absorbance spectrum of a K2MnS2H4O10 single crystal. b What Does High Absorbance Mean In Spectrophotometry Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. The standard curve is generated by. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Higher absorbance indicates. What Does High Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Fundamentals of Spectrophotometry PowerPoint Presentation, free What Does High Absorbance Mean In Spectrophotometry The standard curve is generated by. For example, techniques like the bca assay allow scientists to estimate the. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. Absorption occurs when the energy of the light matches the energy needed. What Does High Absorbance Mean In Spectrophotometry.
From exoraqjxh.blob.core.windows.net
Spectrophotometry Graph at Rucker blog What Does High Absorbance Mean In Spectrophotometry Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. Higher absorbance indicates stronger absorption.. What Does High Absorbance Mean In Spectrophotometry.
From ibsen.com
Absorption spectroscopy Ibsen Photonics What Does High Absorbance Mean In Spectrophotometry The second step of the process is to generate a standard curve. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Transmittance is the quantity of light that passes through a. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. Higher absorbance indicates stronger. What Does High Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Spectrophotometry Basics PowerPoint Presentation, free download What Does High Absorbance Mean In Spectrophotometry Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. The standard curve is generated by. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the. What Does High Absorbance Mean In Spectrophotometry.
From namrataheda.blogspot.kr
B for Biology Spectrophotometry Atomic Absorption Spectrophotometry What Does High Absorbance Mean In Spectrophotometry Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. Transmittance is the quantity of light that passes through a. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. The wavelength that has the highest absorbance in the spectrum is \(\lambda\). What Does High Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download What Does High Absorbance Mean In Spectrophotometry Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. Transmittance is the quantity of light that passes through a. For example, techniques like the bca assay allow scientists to estimate the. The second step of the process is to generate a standard curve. The standard curve is generated by. The wavelength that. What Does High Absorbance Mean In Spectrophotometry.
From www.researchgate.net
Graph with absorption spectrum from UVVis spectrophotometer and the What Does High Absorbance Mean In Spectrophotometry The second step of the process is to generate a standard curve. Higher absorbance indicates stronger absorption. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. The standard curve is generated by. For example, techniques like the bca assay allow scientists to estimate the. Absorbance (a), also known. What Does High Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download What Does High Absorbance Mean In Spectrophotometry For example, techniques like the bca assay allow scientists to estimate the. The second step of the process is to generate a standard curve. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. The standard curve is generated by. Transmittance is the quantity of light that passes through. What Does High Absorbance Mean In Spectrophotometry.
From mavink.com
Absorbance Color Wheel What Does High Absorbance Mean In Spectrophotometry Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. The second step of the process is to generate a standard curve. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. Transmittance is the quantity of light that passes through a.. What Does High Absorbance Mean In Spectrophotometry.
From www.vernier.com
Decoding Your Absorbance Readings Vernier What Does High Absorbance Mean In Spectrophotometry The standard curve is generated by. Transmittance is the quantity of light that passes through a. For example, techniques like the bca assay allow scientists to estimate the. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. The second. What Does High Absorbance Mean In Spectrophotometry.
From www.jasco-global.com
Principles of UV/vis spectroscopy (7) Bandwidth JASCO Global What Does High Absorbance Mean In Spectrophotometry For example, techniques like the bca assay allow scientists to estimate the. Transmittance is the quantity of light that passes through a. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. The standard curve is generated by. Higher absorbance indicates stronger absorption. Absorbance is a measure of how much infrared radiation is. What Does High Absorbance Mean In Spectrophotometry.
From dxolcsxuo.blob.core.windows.net
How Does A Spectrometer Physically Measure An Absorbance at Casey What Does High Absorbance Mean In Spectrophotometry Higher absorbance indicates stronger absorption. For example, techniques like the bca assay allow scientists to estimate the. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. The standard curve is generated by. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Absorbance (a), also. What Does High Absorbance Mean In Spectrophotometry.
From www.horiba.com
吸光度 What Does High Absorbance Mean In Spectrophotometry For example, techniques like the bca assay allow scientists to estimate the. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. Transmittance is the quantity of light that passes through a. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution.. What Does High Absorbance Mean In Spectrophotometry.
From rheolution.com
Understanding Absorbance at Specific Wavelengths What Does High Absorbance Mean In Spectrophotometry Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. The standard curve is generated by. Transmittance is the quantity of light that passes through a. Absorption occurs when the energy of the light matches the. What Does High Absorbance Mean In Spectrophotometry.
From conductscience.com
Spectrophotometer Conduct Science What Does High Absorbance Mean In Spectrophotometry Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. For example, techniques like the bca assay allow scientists to estimate the. Transmittance is the quantity of light that passes through a. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution.. What Does High Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Fundamentals of Spectrophotometry PowerPoint Presentation, free What Does High Absorbance Mean In Spectrophotometry The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. The standard curve is generated by. For example, techniques. What Does High Absorbance Mean In Spectrophotometry.
From www.researchgate.net
Absorbance spectra of 10 and 11. Download Scientific Diagram What Does High Absorbance Mean In Spectrophotometry Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. Higher absorbance indicates stronger absorption. The standard curve is generated by. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. The second step of the process is to generate a standard curve. Absorbance is a. What Does High Absorbance Mean In Spectrophotometry.
From loezonihm.blob.core.windows.net
What Is The Relationship Between Absorbance Transmittance And The Color What Does High Absorbance Mean In Spectrophotometry Higher absorbance indicates stronger absorption. The second step of the process is to generate a standard curve. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. Transmittance is the quantity of light that passes through a. The standard curve is generated by. For example, techniques like the bca assay allow scientists to. What Does High Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download What Does High Absorbance Mean In Spectrophotometry Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. The standard curve is generated by. For example, techniques like the bca assay allow scientists to estimate the. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Transmittance is the quantity of light that passes through a. Higher absorbance. What Does High Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Fundamentals of Spectrophotometry PowerPoint Presentation, free What Does High Absorbance Mean In Spectrophotometry For example, techniques like the bca assay allow scientists to estimate the. Higher absorbance indicates stronger absorption. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. Absorption occurs when the energy of the light matches the energy needed to. What Does High Absorbance Mean In Spectrophotometry.
From namrataheda.blogspot.com
B for Biology Spectrophotometry UVVisible Spectrophotometry What Does High Absorbance Mean In Spectrophotometry The standard curve is generated by. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. The second step of the process is to generate a standard curve. Transmittance is the quantity. What Does High Absorbance Mean In Spectrophotometry.
From www.linquip.com
Beginners Guide What Is a Spectrophotometer Industrial Manufacturing What Does High Absorbance Mean In Spectrophotometry The second step of the process is to generate a standard curve. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Transmittance is the quantity of light that passes through a. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. Absorption occurs when the energy of the light. What Does High Absorbance Mean In Spectrophotometry.
From www.researchgate.net
Calibration of spectrophotometer by measuring absorbance of CuSO4 What Does High Absorbance Mean In Spectrophotometry Transmittance is the quantity of light that passes through a. The second step of the process is to generate a standard curve. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. Absorbance is a measure of how much infrared. What Does High Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Fundamentals of Spectrophotometry PowerPoint Presentation, free What Does High Absorbance Mean In Spectrophotometry Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. Higher absorbance indicates stronger absorption. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. The standard curve is. What Does High Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download What Does High Absorbance Mean In Spectrophotometry Higher absorbance indicates stronger absorption. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. For example, techniques like the bca assay allow scientists to estimate the. Transmittance is the quantity of. What Does High Absorbance Mean In Spectrophotometry.
From scienceinfo.com
Atomic Absorption Spectrophotometry Principle, Parts, Uses What Does High Absorbance Mean In Spectrophotometry Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. Transmittance is the quantity of light that passes through a. The standard curve is generated by. For example, techniques like the bca assay allow scientists to estimate the. Absorbance is a measure of how much infrared radiation is absorbed by a sample at. What Does High Absorbance Mean In Spectrophotometry.
From www.researchgate.net
The relationship between absorbance measured on a spectrophotometer What Does High Absorbance Mean In Spectrophotometry Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. The standard curve is generated by. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the. What Does High Absorbance Mean In Spectrophotometry.
From www.scribd.com
Spectrophotometry.ppt 18.07.08 Absorbance Spectrophotometry What Does High Absorbance Mean In Spectrophotometry Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. Absorbance is a measure of how much infrared radiation. What Does High Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download What Does High Absorbance Mean In Spectrophotometry Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Higher absorbance indicates stronger absorption. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. The second step of. What Does High Absorbance Mean In Spectrophotometry.
From www.researchgate.net
Analysis of UV Absorbance spectra? What Does High Absorbance Mean In Spectrophotometry The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Absorption occurs when the energy of the light matches the energy needed to move electrons within the molecules of the material. For example, techniques like the bca assay allow scientists to estimate the. Absorbance is a measure of how much infrared radiation is absorbed by a sample. What Does High Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID6911139 What Does High Absorbance Mean In Spectrophotometry Transmittance is the quantity of light that passes through a. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. The second step of the process is to generate a standard curve. For example, techniques like the bca assay allow scientists to estimate the. Absorption occurs when the energy of the light matches. What Does High Absorbance Mean In Spectrophotometry.
From shaniyaukung.blogspot.com
What Is Absorbance In Spectrophotometer What Does High Absorbance Mean In Spectrophotometry The second step of the process is to generate a standard curve. The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Transmittance is the quantity of light that passes through a. The standard curve is generated by. Higher absorbance indicates stronger absorption. Absorbance is a measure of how much infrared radiation is absorbed by a sample. What Does High Absorbance Mean In Spectrophotometry.
From www.researchgate.net
UVVis spectrophotometer absorbance and reflectance spectrums of ZnO What Does High Absorbance Mean In Spectrophotometry Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. The second step of the process is to generate a standard curve. Transmittance is the quantity of light that passes through a. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. Absorption occurs when the. What Does High Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Fundamentals of Spectrophotometry PowerPoint Presentation, free What Does High Absorbance Mean In Spectrophotometry The wavelength that has the highest absorbance in the spectrum is \(\lambda\) max. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. Absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. Absorption occurs when the energy of the light matches the energy needed to. What Does High Absorbance Mean In Spectrophotometry.