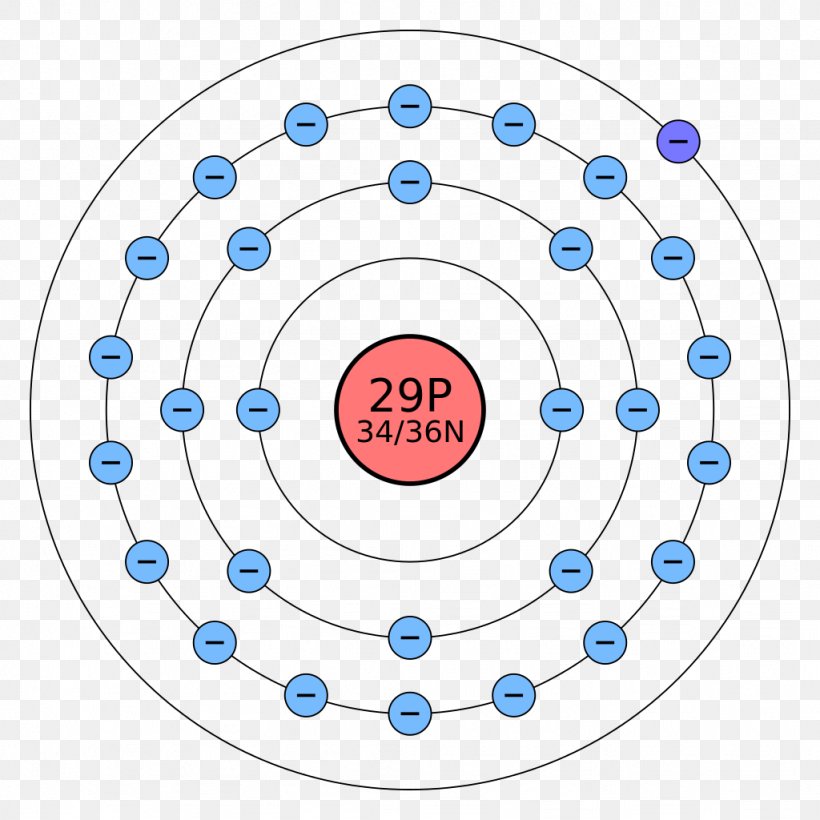
Zinc Electron Shell Arrangement . 119 rows the m shell contains 3s, 3p, and 3d, and can carry 18 electrons. View rotating bohr models for all 118 elements. Get a free hd image of the periodic. Access detailed info on all elements: In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. The first shell (closest to the nucleus) contains 2. There are two valence electrons in the outer shell of the zinc. Zinc's location reflects the fact that is has a completely filled third electron shell. The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically focusing on the arrangement of electrons in the outermost energy level, also. The n shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. The distribution of electrons in shells in a zinc atom can be represented as follows: Atomic mass, electron configurations, charges, and more.
from antasyaalinda.blogspot.com
119 rows the m shell contains 3s, 3p, and 3d, and can carry 18 electrons. Get a free hd image of the periodic. View rotating bohr models for all 118 elements. In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. Atomic mass, electron configurations, charges, and more. Zinc's location reflects the fact that is has a completely filled third electron shell. Access detailed info on all elements: The distribution of electrons in shells in a zinc atom can be represented as follows: The first shell (closest to the nucleus) contains 2. The n shell containing 4s, 4d, 4p and 4f, can carry 32 electrons.
Diagram Of Zinc Atom
Zinc Electron Shell Arrangement Zinc's location reflects the fact that is has a completely filled third electron shell. View rotating bohr models for all 118 elements. The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically focusing on the arrangement of electrons in the outermost energy level, also. Atomic mass, electron configurations, charges, and more. The distribution of electrons in shells in a zinc atom can be represented as follows: In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. 119 rows the m shell contains 3s, 3p, and 3d, and can carry 18 electrons. Zinc's location reflects the fact that is has a completely filled third electron shell. The n shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. Access detailed info on all elements: Get a free hd image of the periodic. There are two valence electrons in the outer shell of the zinc. The first shell (closest to the nucleus) contains 2.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Shell Arrangement The n shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. Access detailed info on all elements: View rotating bohr models for all 118 elements. In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. 119 rows the m shell contains 3s, 3p, and 3d, and can carry 18. Zinc Electron Shell Arrangement.
From material-properties.org
Zinc Periodic Table and Atomic Properties Zinc Electron Shell Arrangement Zinc's location reflects the fact that is has a completely filled third electron shell. There are two valence electrons in the outer shell of the zinc. Get a free hd image of the periodic. Atomic mass, electron configurations, charges, and more. The distribution of electrons in shells in a zinc atom can be represented as follows: Access detailed info on. Zinc Electron Shell Arrangement.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Shell Arrangement The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically focusing on the arrangement of electrons in the outermost energy level, also. Access detailed info on all elements: The distribution of electrons in shells in a zinc atom can be represented as follows: View rotating bohr models for all 118 elements. In 1913, niels. Zinc Electron Shell Arrangement.
From www.britannica.com
Electron shell Definition & Facts Britannica Zinc Electron Shell Arrangement View rotating bohr models for all 118 elements. In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. The first shell (closest to the nucleus) contains 2. Access detailed info on all elements: 119 rows the m shell contains 3s, 3p, and 3d, and can carry 18 electrons. Atomic mass, electron. Zinc Electron Shell Arrangement.
From stock.adobe.com
Zinc electron configuration and its propertiesvector illustration Zinc Electron Shell Arrangement Atomic mass, electron configurations, charges, and more. Access detailed info on all elements: The distribution of electrons in shells in a zinc atom can be represented as follows: Zinc's location reflects the fact that is has a completely filled third electron shell. View rotating bohr models for all 118 elements. There are two valence electrons in the outer shell of. Zinc Electron Shell Arrangement.
From www.youtube.com
zinc electronic configuration How to Write Zinc electronic Zinc Electron Shell Arrangement 119 rows the m shell contains 3s, 3p, and 3d, and can carry 18 electrons. Atomic mass, electron configurations, charges, and more. The first shell (closest to the nucleus) contains 2. Zinc's location reflects the fact that is has a completely filled third electron shell. The distribution of electrons in shells in a zinc atom can be represented as follows:. Zinc Electron Shell Arrangement.
From www.schoolmykids.com
Zinc (Zn) Element Information, Facts, Properties, Uses Periodic Zinc Electron Shell Arrangement The distribution of electrons in shells in a zinc atom can be represented as follows: View rotating bohr models for all 118 elements. There are two valence electrons in the outer shell of the zinc. Access detailed info on all elements: The first shell (closest to the nucleus) contains 2. The n shell containing 4s, 4d, 4p and 4f, can. Zinc Electron Shell Arrangement.
From antasyaalinda.blogspot.com
Diagram Of Zinc Atom Zinc Electron Shell Arrangement There are two valence electrons in the outer shell of the zinc. 119 rows the m shell contains 3s, 3p, and 3d, and can carry 18 electrons. The n shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. Zinc's location reflects the fact that is has a completely filled third electron shell. The distribution of electrons in shells. Zinc Electron Shell Arrangement.
From www.youtube.com
Electron Configuration of Zinc Zn Lesson YouTube Zinc Electron Shell Arrangement 119 rows the m shell contains 3s, 3p, and 3d, and can carry 18 electrons. Get a free hd image of the periodic. The n shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. Access detailed info on all elements: View rotating bohr models for all 118 elements. The first shell (closest to the nucleus) contains 2. There. Zinc Electron Shell Arrangement.
From dxokssylp.blob.core.windows.net
Zinc Number Of Electrons In Shells at Fredrick Williams blog Zinc Electron Shell Arrangement Get a free hd image of the periodic. The first shell (closest to the nucleus) contains 2. Zinc's location reflects the fact that is has a completely filled third electron shell. The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically focusing on the arrangement of electrons in the outermost energy level, also. The. Zinc Electron Shell Arrangement.
From periodiske-system.dk
Zinc Zinc Electron Shell Arrangement The first shell (closest to the nucleus) contains 2. Get a free hd image of the periodic. Zinc's location reflects the fact that is has a completely filled third electron shell. In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. Atomic mass, electron configurations, charges, and more. The n shell. Zinc Electron Shell Arrangement.
From fyonxdqvl.blob.core.windows.net
Zinc Number Of Electrons In Outer Ring at Travis Taylor blog Zinc Electron Shell Arrangement The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically focusing on the arrangement of electrons in the outermost energy level, also. The distribution of electrons in shells in a zinc atom can be represented as follows: Zinc's location reflects the fact that is has a completely filled third electron shell. Get a free. Zinc Electron Shell Arrangement.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Shell Arrangement In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. The distribution of electrons in shells in a zinc atom can be represented as follows: There are two valence electrons in the outer shell of the zinc. 119 rows the m shell contains 3s, 3p, and 3d, and can carry 18. Zinc Electron Shell Arrangement.
From periodictable.me
Zinc Electron Configuration (Zn) with Orbital Diagram Zinc Electron Shell Arrangement View rotating bohr models for all 118 elements. There are two valence electrons in the outer shell of the zinc. Zinc's location reflects the fact that is has a completely filled third electron shell. Access detailed info on all elements: The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically focusing on the arrangement. Zinc Electron Shell Arrangement.
From revision.my
2.4.1 Electron Arrangement Revision.my Zinc Electron Shell Arrangement 119 rows the m shell contains 3s, 3p, and 3d, and can carry 18 electrons. In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. The n shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. View rotating bohr models for all 118 elements. Get a free hd image. Zinc Electron Shell Arrangement.
From www.alamy.com
Zn Zinc, Periodic Table of the Elements, Shell Structure of Zinc Zinc Electron Shell Arrangement The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically focusing on the arrangement of electrons in the outermost energy level, also. In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. The first shell (closest to the nucleus) contains 2. There are two valence. Zinc Electron Shell Arrangement.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Zinc Electron Shell Arrangement The n shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. 119 rows the m shell contains 3s, 3p, and 3d, and can carry 18 electrons. Access detailed info on all elements: Get a free hd image of the. Zinc Electron Shell Arrangement.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Shell Arrangement In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. Access detailed info on all elements: The first shell (closest to the nucleus) contains 2. There are two valence electrons in the outer shell of the zinc. View rotating bohr models for all 118 elements. Zinc's location reflects the fact that. Zinc Electron Shell Arrangement.
From www.schoolmykids.com
Zinc (Zn) Element Information, Facts, Properties, Uses Periodic Zinc Electron Shell Arrangement The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically focusing on the arrangement of electrons in the outermost energy level, also. The n shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. Get a free hd image of the periodic. In 1913, niels bohr proposed a model of the atom, giving. Zinc Electron Shell Arrangement.
From www.alamy.com
Zinc Atom Shell Stock Vector Image & Art Alamy Zinc Electron Shell Arrangement View rotating bohr models for all 118 elements. The n shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. Atomic mass, electron configurations, charges, and more. Access detailed info on all elements: Get a free hd image of the periodic. There are two valence electrons in the outer shell of the zinc. 119 rows the m shell contains. Zinc Electron Shell Arrangement.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Shell Arrangement The first shell (closest to the nucleus) contains 2. View rotating bohr models for all 118 elements. Zinc's location reflects the fact that is has a completely filled third electron shell. Get a free hd image of the periodic. The n shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. Access detailed info on all elements: There are. Zinc Electron Shell Arrangement.
From www.alamy.com
Symbol and electron diagram for Zinc illustration Stock Vector Image Zinc Electron Shell Arrangement 119 rows the m shell contains 3s, 3p, and 3d, and can carry 18 electrons. The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically focusing on the arrangement of electrons in the outermost energy level, also. The n shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. Atomic mass, electron configurations,. Zinc Electron Shell Arrangement.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Shell Arrangement The distribution of electrons in shells in a zinc atom can be represented as follows: In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. There are two valence electrons in the outer shell of the zinc. The n shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. Zinc's. Zinc Electron Shell Arrangement.
From en.academic.ru
Zinc Zinc Electron Shell Arrangement The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically focusing on the arrangement of electrons in the outermost energy level, also. Atomic mass, electron configurations, charges, and more. In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. There are two valence electrons in. Zinc Electron Shell Arrangement.
From www.shutterstock.com
Zinc Zn Element Sphere Electron Shell Stock Vector (Royalty Free Zinc Electron Shell Arrangement In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. The distribution of electrons in shells in a zinc atom can be represented as follows: Get a free hd image of the periodic. Access detailed info on all elements: View rotating bohr models for all 118 elements. There are two valence. Zinc Electron Shell Arrangement.
From exoniwkzl.blob.core.windows.net
Zinc With 28 Electrons Charge at Scott Kauffman blog Zinc Electron Shell Arrangement Atomic mass, electron configurations, charges, and more. Get a free hd image of the periodic. Access detailed info on all elements: Zinc's location reflects the fact that is has a completely filled third electron shell. The valence orbital diagram is a graphical representation of the electron configuration of an atom, specifically focusing on the arrangement of electrons in the outermost. Zinc Electron Shell Arrangement.
From www.sciencephoto.com
Zinc electron configuration Stock Image C029/5029 Science Photo Zinc Electron Shell Arrangement In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. The first shell (closest to the nucleus) contains 2. Zinc's location reflects the fact that is has a completely filled third electron shell. There are two valence electrons in the outer shell of the zinc. 119 rows the m shell contains. Zinc Electron Shell Arrangement.
From periodictableguide.com
Zinc (Zn) Periodic Table (Element Information & More) Zinc Electron Shell Arrangement Access detailed info on all elements: 119 rows the m shell contains 3s, 3p, and 3d, and can carry 18 electrons. Zinc's location reflects the fact that is has a completely filled third electron shell. Atomic mass, electron configurations, charges, and more. The first shell (closest to the nucleus) contains 2. There are two valence electrons in the outer shell. Zinc Electron Shell Arrangement.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Shell Arrangement Atomic mass, electron configurations, charges, and more. In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. There are two valence electrons in the outer shell of the zinc. Get a free hd image of the periodic. 119 rows the m shell contains 3s, 3p, and 3d, and can carry 18. Zinc Electron Shell Arrangement.
From www.animalia-life.club
Electron Shell Diagram Zinc Electron Shell Arrangement In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. Get a free hd image of the periodic. View rotating bohr models for all 118 elements. Atomic mass, electron configurations, charges, and more. The distribution of electrons in shells in a zinc atom can be represented as follows: Zinc's location reflects. Zinc Electron Shell Arrangement.
From valenceelectrons.com
How Many Valence Electrons Does Zinc (Zn) Have? Zinc Electron Shell Arrangement The n shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. Get a free hd image of the periodic. Atomic mass, electron configurations, charges, and more. There are two valence electrons in the outer shell of the zinc. View rotating bohr models for all 118 elements. The distribution of electrons in shells in a zinc atom can be. Zinc Electron Shell Arrangement.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Shell Arrangement The first shell (closest to the nucleus) contains 2. View rotating bohr models for all 118 elements. In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. Atomic mass, electron configurations, charges, and more. Get a free hd image of the periodic. The distribution of electrons in shells in a zinc. Zinc Electron Shell Arrangement.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Shell Arrangement The first shell (closest to the nucleus) contains 2. The n shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. The distribution of electrons in shells in a zinc atom can be represented as follows: Zinc's location reflects the fact that is has a completely filled third electron shell. View rotating bohr models for all 118 elements. The. Zinc Electron Shell Arrangement.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Zinc Electron Shell Arrangement The first shell (closest to the nucleus) contains 2. 119 rows the m shell contains 3s, 3p, and 3d, and can carry 18 electrons. Zinc's location reflects the fact that is has a completely filled third electron shell. Atomic mass, electron configurations, charges, and more. Get a free hd image of the periodic. View rotating bohr models for all 118. Zinc Electron Shell Arrangement.
From www.sciencephoto.com
Zinc, atomic structure Stock Image C013/1553 Science Photo Library Zinc Electron Shell Arrangement Get a free hd image of the periodic. In 1913, niels bohr proposed a model of the atom, giving the arrangement of electrons in their sequential orbits. The distribution of electrons in shells in a zinc atom can be represented as follows: There are two valence electrons in the outer shell of the zinc. The n shell containing 4s, 4d,. Zinc Electron Shell Arrangement.