What Is Meant By Heating Value . 1 btu/lb = 2326.1 j/kg = 0.55556 kcal/kg. higher heating value (hhv) is defined as the amount of heat released by the unit mass or volume of fuel (initially at 25 °c) once it is. 1 j/kg = 0.00043 btu/lb = 2.39x10. Higher calorific value, also known as gross calorific value. Heating value of fuel is defined as the energy released per unit mass of fuel in a complete. Calorific value) of a substance is used to indicate how much heat will be released by the process of. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower heating. calorific value, also known as heating value or energy content, is a measure of the energy released during the combustion of a. heat of combustion is often categorized into the following two types: heating values of combustion. the heat value (a.k.a. 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy ), is the. Common units for heating value:
from www.slideserve.com
1 btu/lb = 2326.1 j/kg = 0.55556 kcal/kg. heat of combustion is often categorized into the following two types: Common units for heating value: Higher calorific value, also known as gross calorific value. 1 j/kg = 0.00043 btu/lb = 2.39x10. Heating value of fuel is defined as the energy released per unit mass of fuel in a complete. higher heating value (hhv) is defined as the amount of heat released by the unit mass or volume of fuel (initially at 25 °c) once it is. Calorific value) of a substance is used to indicate how much heat will be released by the process of. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower heating. heating values of combustion.
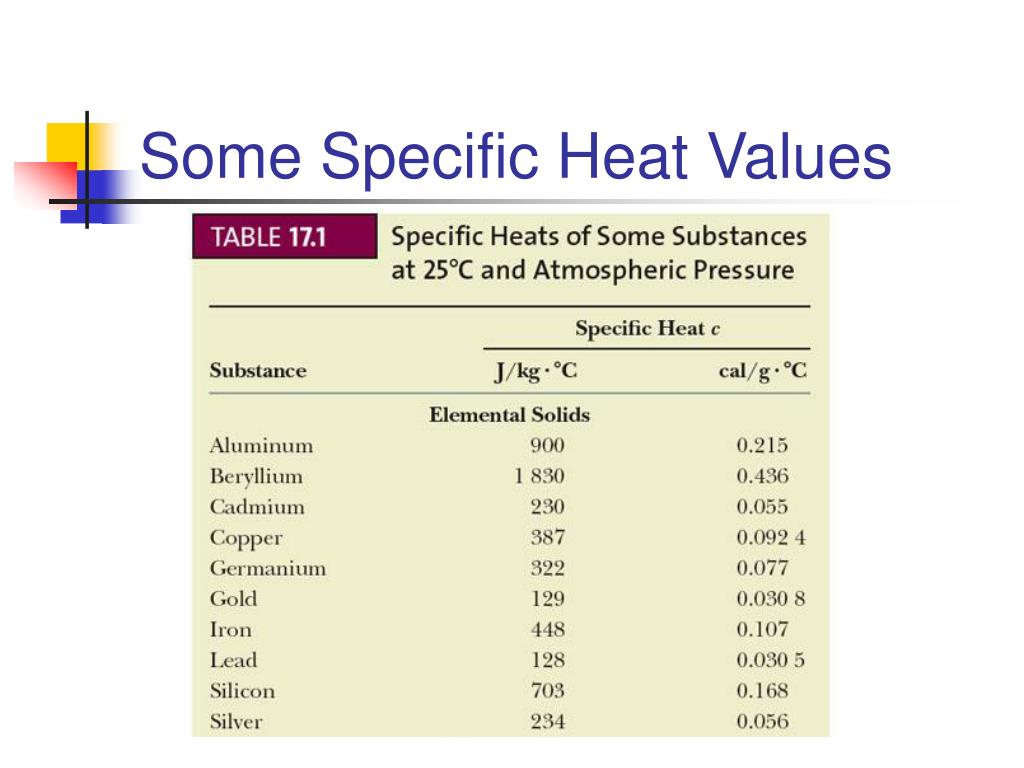
PPT Chapter 17 PowerPoint Presentation, free download ID1792465
What Is Meant By Heating Value Calorific value) of a substance is used to indicate how much heat will be released by the process of. the heat value (a.k.a. Heating value of fuel is defined as the energy released per unit mass of fuel in a complete. 1 btu/lb = 2326.1 j/kg = 0.55556 kcal/kg. Calorific value) of a substance is used to indicate how much heat will be released by the process of. Higher calorific value, also known as gross calorific value. 1 j/kg = 0.00043 btu/lb = 2.39x10. heating values of combustion. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower heating. Common units for heating value: heat of combustion is often categorized into the following two types: 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy ), is the. higher heating value (hhv) is defined as the amount of heat released by the unit mass or volume of fuel (initially at 25 °c) once it is. calorific value, also known as heating value or energy content, is a measure of the energy released during the combustion of a.
From www.researchgate.net
Waste heat values obtained from different sources at low, medium and What Is Meant By Heating Value 1 btu/lb = 2326.1 j/kg = 0.55556 kcal/kg. higher heating value (hhv) is defined as the amount of heat released by the unit mass or volume of fuel (initially at 25 °c) once it is. the heat value (a.k.a. heating values of combustion. 1 j/kg = 0.00043 btu/lb = 2.39x10. Higher calorific value, also known as gross. What Is Meant By Heating Value.
From www.slideserve.com
PPT Change of State PowerPoint Presentation, free download ID6691426 What Is Meant By Heating Value 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy ), is the. heat of combustion is often categorized into the following two types: heating values of combustion. 1 btu/lb = 2326.1 j/kg = 0.55556 kcal/kg. 1 j/kg = 0.00043 btu/lb = 2.39x10. Calorific value). What Is Meant By Heating Value.
From www.slideserve.com
PPT Energy Measurement Concepts PowerPoint Presentation, free What Is Meant By Heating Value Common units for heating value: heat of combustion is often categorized into the following two types: Higher calorific value, also known as gross calorific value. heating values of combustion. Heating value of fuel is defined as the energy released per unit mass of fuel in a complete. higher heating value (hhv) is defined as the amount of. What Is Meant By Heating Value.
From www.slideserve.com
PPT Lecture 3. Fuel Cell Thermodynamics PowerPoint Presentation, free What Is Meant By Heating Value Higher calorific value, also known as gross calorific value. Common units for heating value: 1 j/kg = 0.00043 btu/lb = 2.39x10. heating values of combustion. Heating value of fuel is defined as the energy released per unit mass of fuel in a complete. the heat value (a.k.a. Calorific value) of a substance is used to indicate how much. What Is Meant By Heating Value.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID1792465 What Is Meant By Heating Value Heating value of fuel is defined as the energy released per unit mass of fuel in a complete. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower heating. the heat value (a.k.a. 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or. What Is Meant By Heating Value.
From mavink.com
Specific Heat Table Of Values What Is Meant By Heating Value Higher calorific value, also known as gross calorific value. Common units for heating value: Calorific value) of a substance is used to indicate how much heat will be released by the process of. 1 btu/lb = 2326.1 j/kg = 0.55556 kcal/kg. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower heating. . What Is Meant By Heating Value.
From www.researchgate.net
Summary of Predictive Heating Value Results Download Table What Is Meant By Heating Value Heating value of fuel is defined as the energy released per unit mass of fuel in a complete. heat of combustion is often categorized into the following two types: Calorific value) of a substance is used to indicate how much heat will be released by the process of. Higher calorific value, also known as gross calorific value. 84. What Is Meant By Heating Value.
From www.slideserve.com
PPT Lecture Objectives PowerPoint Presentation, free download ID What Is Meant By Heating Value the heat value (a.k.a. Heating value of fuel is defined as the energy released per unit mass of fuel in a complete. 1 j/kg = 0.00043 btu/lb = 2.39x10. Calorific value) of a substance is used to indicate how much heat will be released by the process of. Higher calorific value, also known as gross calorific value. higher. What Is Meant By Heating Value.
From www.researchgate.net
Heating value of several fuels. Download Table What Is Meant By Heating Value Heating value of fuel is defined as the energy released per unit mass of fuel in a complete. heating values of combustion. 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy ), is the. Higher calorific value, also known as gross calorific value. 1 j/kg. What Is Meant By Heating Value.
From haipernews.com
How To Calculate Specific Heat Capacity Without Q Haiper What Is Meant By Heating Value calorific value, also known as heating value or energy content, is a measure of the energy released during the combustion of a. higher heating value (hhv) is defined as the amount of heat released by the unit mass or volume of fuel (initially at 25 °c) once it is. heat of combustion is often categorized into the. What Is Meant By Heating Value.
From www.researchgate.net
Fuel heating value to calculate furnace Watt power Download Table What Is Meant By Heating Value 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy ), is the. Calorific value) of a substance is used to indicate how much heat will be released by the process of. higher heating value (hhv) is defined as the amount of heat released by the. What Is Meant By Heating Value.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID5988619 What Is Meant By Heating Value The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower heating. heating values of combustion. 1 j/kg = 0.00043 btu/lb = 2.39x10. Calorific value) of a substance is used to indicate how much heat will be released by the process of. 84 rows the heating value (or energy value or calorific. What Is Meant By Heating Value.
From audran-frock.blogspot.com
Heating Value Ghv Gross Heating Value By Acronymsandslang Com What Is Meant By Heating Value heat of combustion is often categorized into the following two types: 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy ), is the. Heating value of fuel is defined as the energy released per unit mass of fuel in a complete. Common units for heating. What Is Meant By Heating Value.
From www.youtube.com
Fuel Cell (0603) Thermodynamics Heating Value YouTube What Is Meant By Heating Value higher heating value (hhv) is defined as the amount of heat released by the unit mass or volume of fuel (initially at 25 °c) once it is. heating values of combustion. the heat value (a.k.a. 1 btu/lb = 2326.1 j/kg = 0.55556 kcal/kg. heat of combustion is often categorized into the following two types: 84. What Is Meant By Heating Value.
From abramsrewing.blogspot.com
Specific Heat Capacity Definition AbramsrEwing What Is Meant By Heating Value heat of combustion is often categorized into the following two types: the heat value (a.k.a. 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy ), is the. heating values of combustion. Calorific value) of a substance is used to indicate how much heat. What Is Meant By Heating Value.
From www.youtube.com
Mechanical Engineering Thermodynamics Lec 32, pt 3 of 3 Heating What Is Meant By Heating Value The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower heating. 1 j/kg = 0.00043 btu/lb = 2.39x10. heating values of combustion. Heating value of fuel is defined as the energy released per unit mass of fuel in a complete. Calorific value) of a substance is used to indicate how much heat. What Is Meant By Heating Value.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID4499064 What Is Meant By Heating Value higher heating value (hhv) is defined as the amount of heat released by the unit mass or volume of fuel (initially at 25 °c) once it is. heating values of combustion. 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy ), is the. 1. What Is Meant By Heating Value.
From www.researchgate.net
Lower heating values of fuels and species involved in the EROI What Is Meant By Heating Value Calorific value) of a substance is used to indicate how much heat will be released by the process of. Heating value of fuel is defined as the energy released per unit mass of fuel in a complete. 1 j/kg = 0.00043 btu/lb = 2.39x10. Higher calorific value, also known as gross calorific value. calorific value, also known as heating. What Is Meant By Heating Value.
From www.researchgate.net
the heating value of diesel, neat biodiesel and their blends in MJ/Kg What Is Meant By Heating Value Common units for heating value: 1 btu/lb = 2326.1 j/kg = 0.55556 kcal/kg. the heat value (a.k.a. higher heating value (hhv) is defined as the amount of heat released by the unit mass or volume of fuel (initially at 25 °c) once it is. The heat of combustion for fuels is expressed as the hhv (higher heating value). What Is Meant By Heating Value.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID6948130 What Is Meant By Heating Value 1 j/kg = 0.00043 btu/lb = 2.39x10. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower heating. Calorific value) of a substance is used to indicate how much heat will be released by the process of. heat of combustion is often categorized into the following two types: the heat value. What Is Meant By Heating Value.
From zuoti.pro
4. What is the average specific heat capacity of the unknown metal in What Is Meant By Heating Value The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower heating. Common units for heating value: Heating value of fuel is defined as the energy released per unit mass of fuel in a complete. heating values of combustion. higher heating value (hhv) is defined as the amount of heat released by. What Is Meant By Heating Value.
From www.researchgate.net
The lower heating values (LHVs) of NH 3 H 2 and NH 3 H 2 N 2 What Is Meant By Heating Value 1 btu/lb = 2326.1 j/kg = 0.55556 kcal/kg. higher heating value (hhv) is defined as the amount of heat released by the unit mass or volume of fuel (initially at 25 °c) once it is. Common units for heating value: calorific value, also known as heating value or energy content, is a measure of the energy released during. What Is Meant By Heating Value.
From www.teachoo.com
For any substance, why does the temperature remain constant during the What Is Meant By Heating Value 1 btu/lb = 2326.1 j/kg = 0.55556 kcal/kg. higher heating value (hhv) is defined as the amount of heat released by the unit mass or volume of fuel (initially at 25 °c) once it is. Common units for heating value: the heat value (a.k.a. heat of combustion is often categorized into the following two types: Higher calorific. What Is Meant By Heating Value.
From www.numerade.com
SOLVED Table 4.2 SPECIFIC HEATS OF COMMON SUBSTANCES Material What Is Meant By Heating Value The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower heating. 1 j/kg = 0.00043 btu/lb = 2.39x10. the heat value (a.k.a. calorific value, also known as heating value or energy content, is a measure of the energy released during the combustion of a. Higher calorific value, also known as gross. What Is Meant By Heating Value.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6948711 What Is Meant By Heating Value 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy ), is the. the heat value (a.k.a. heating values of combustion. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower heating. heat of combustion is often. What Is Meant By Heating Value.
From heatinggondon.blogspot.com
Heating Heating Value Of Hydrogen What Is Meant By Heating Value Calorific value) of a substance is used to indicate how much heat will be released by the process of. 1 btu/lb = 2326.1 j/kg = 0.55556 kcal/kg. 1 j/kg = 0.00043 btu/lb = 2.39x10. Common units for heating value: heat of combustion is often categorized into the following two types: calorific value, also known as heating value or. What Is Meant By Heating Value.
From energyknowledgebase.com
Heating value · Energy KnowledgeBase What Is Meant By Heating Value Higher calorific value, also known as gross calorific value. calorific value, also known as heating value or energy content, is a measure of the energy released during the combustion of a. Common units for heating value: higher heating value (hhv) is defined as the amount of heat released by the unit mass or volume of fuel (initially at. What Is Meant By Heating Value.
From www.mathscinotes.com
CO2 Generation By Fuel Per Million BTUs of Heat Math Encounters Blog What Is Meant By Heating Value 1 btu/lb = 2326.1 j/kg = 0.55556 kcal/kg. Calorific value) of a substance is used to indicate how much heat will be released by the process of. Higher calorific value, also known as gross calorific value. Common units for heating value: 1 j/kg = 0.00043 btu/lb = 2.39x10. Heating value of fuel is defined as the energy released per unit. What Is Meant By Heating Value.
From energyknowledgebase.com
Heating value · Energy KnowledgeBase What Is Meant By Heating Value 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy ), is the. heat of combustion is often categorized into the following two types: 1 j/kg = 0.00043 btu/lb = 2.39x10. Common units for heating value: Calorific value) of a substance is used to indicate how. What Is Meant By Heating Value.
From www.researchgate.net
Heating values for various fuels and MSW components Source Voelker What Is Meant By Heating Value Higher calorific value, also known as gross calorific value. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower heating. heat of combustion is often categorized into the following two types: 1 j/kg = 0.00043 btu/lb = 2.39x10. Heating value of fuel is defined as the energy released per unit mass of. What Is Meant By Heating Value.
From energyknowledgebase.com
Heating value · Energy KnowledgeBase What Is Meant By Heating Value heat of combustion is often categorized into the following two types: Higher calorific value, also known as gross calorific value. 1 btu/lb = 2326.1 j/kg = 0.55556 kcal/kg. Calorific value) of a substance is used to indicate how much heat will be released by the process of. calorific value, also known as heating value or energy content, is. What Is Meant By Heating Value.
From www.researchgate.net
The lower heating values (LHVs) of NH 3 H 2 and NH 3 H 2 N 2 What Is Meant By Heating Value the heat value (a.k.a. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower heating. Calorific value) of a substance is used to indicate how much heat will be released by the process of. Higher calorific value, also known as gross calorific value. higher heating value (hhv) is defined as the. What Is Meant By Heating Value.
From www.researchgate.net
The lower heating values and energy content of the unsorted solid waste What Is Meant By Heating Value calorific value, also known as heating value or energy content, is a measure of the energy released during the combustion of a. Heating value of fuel is defined as the energy released per unit mass of fuel in a complete. the heat value (a.k.a. higher heating value (hhv) is defined as the amount of heat released by. What Is Meant By Heating Value.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID6948130 What Is Meant By Heating Value Calorific value) of a substance is used to indicate how much heat will be released by the process of. Heating value of fuel is defined as the energy released per unit mass of fuel in a complete. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower heating. calorific value, also known. What Is Meant By Heating Value.
From www.youtube.com
Calculating calorific value YouTube What Is Meant By Heating Value calorific value, also known as heating value or energy content, is a measure of the energy released during the combustion of a. 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy ), is the. Higher calorific value, also known as gross calorific value. The heat. What Is Meant By Heating Value.