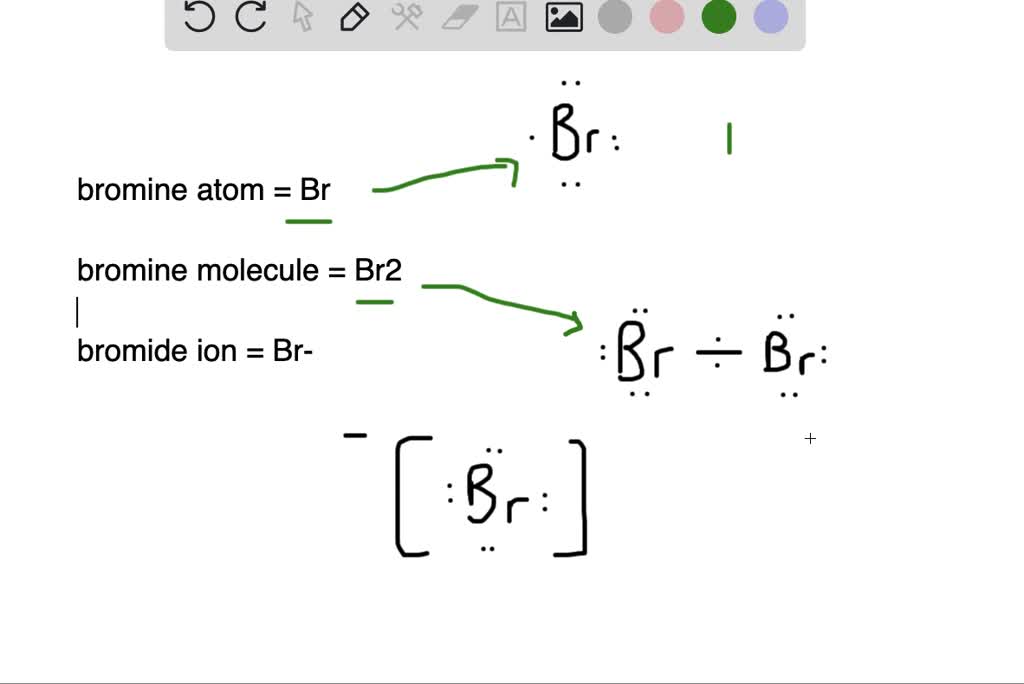
Bromide Anion Charge . For example, iron(ii) has a 2+ charge; This is because bromine has seven electrons in its outer shell, which. It is a conjugate base of a hydrogen. A bromide ion is an electrically charged atom of the element bromine. Bromide is a halide anion and a monoatomic bromine. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. There is one cobalt ion. Predict the charge of monatomic main group elements based on their group. Only one ion of each is needed to.
from ceukyrig.blob.core.windows.net
Only one ion of each is needed to. There is one cobalt ion. Predict the charge of monatomic main group elements based on their group. A bromide ion is an electrically charged atom of the element bromine. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. This is because bromine has seven electrons in its outer shell, which. Bromide is a halide anion and a monoatomic bromine. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. It is a conjugate base of a hydrogen. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions.
Aqueous Zinc Bromide Equation at Heather Spiers blog
Bromide Anion Charge Only one ion of each is needed to. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. For example, iron(ii) has a 2+ charge; A bromide ion is an electrically charged atom of the element bromine. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. It is a conjugate base of a hydrogen. There is one cobalt ion. This is because bromine has seven electrons in its outer shell, which. Predict the charge of monatomic main group elements based on their group. Only one ion of each is needed to. Bromide is a halide anion and a monoatomic bromine.
From www.youtube.com
How to Write the Formula for Lead (II) bromide YouTube Bromide Anion Charge Roman numeral notation indicates charge of ion when element commonly forms more than one ion. A bromide ion is an electrically charged atom of the element bromine. Only one ion of each is needed to. For example, iron(ii) has a 2+ charge; This is because bromine has seven electrons in its outer shell, which. If we look at the ionic. Bromide Anion Charge.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube Bromide Anion Charge Only one ion of each is needed to. Bromide is a halide anion and a monoatomic bromine. For example, iron(ii) has a 2+ charge; If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. A bromide ion is. Bromide Anion Charge.
From www.chegg.com
Solved Suppose of sodium bromide is dissolved in of a Bromide Anion Charge For example, iron(ii) has a 2+ charge; Only one ion of each is needed to. It is a conjugate base of a hydrogen. A bromide ion is an electrically charged atom of the element bromine. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. There is one cobalt ion. If we look at. Bromide Anion Charge.
From www.numerade.com
SOLVED Table 2 Write the symbol charge of each ion in each compound Bromide Anion Charge There is one cobalt ion. Bromide is a halide anion and a monoatomic bromine. Only one ion of each is needed to. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. Roman numeral notation indicates charge of. Bromide Anion Charge.
From chemistrysteps.com
Reactions of Thiols Chemistry Steps Bromide Anion Charge Bromide is a halide anion and a monoatomic bromine. For example, iron(ii) has a 2+ charge; This is because bromine has seven electrons in its outer shell, which. There is one cobalt ion. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. If we look at the ionic compound consisting of lithium ions. Bromide Anion Charge.
From chemistry291.blogspot.com
Magnesium Bromide FormulaFormula for Magnesium Bromide Bromide Anion Charge Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Only one ion of each is needed to. This is because bromine has seven electrons in its outer shell, which. Predict the charge of monatomic main group elements based on their group. Bromide is a halide anion and a monoatomic bromine. A bromide ion. Bromide Anion Charge.
From www.numerade.com
SOLVED Texts File Edit View Insert Format Tools Addon 3 A 7 100 Bromide Anion Charge If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. A bromide ion is an electrically charged atom of the element bromine. Predict the charge of monatomic main group elements based on their group. For example, iron(ii) has. Bromide Anion Charge.
From www.youtube.com
TlBr5 Tellurium Penta Bromide anion Shape Hybridisation VSEPR Bromide Anion Charge Only one ion of each is needed to. A bromide ion is an electrically charged atom of the element bromine. For example, iron(ii) has a 2+ charge; If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. This. Bromide Anion Charge.
From klanyvtvc.blob.core.windows.net
Magnesium Bromide at Reba Craig blog Bromide Anion Charge This is because bromine has seven electrons in its outer shell, which. A bromide ion is an electrically charged atom of the element bromine. For example, iron(ii) has a 2+ charge; It is a conjugate base of a hydrogen. Only one ion of each is needed to. Recognize that metals lose electrons to form cations and that nonmetals gain electrons. Bromide Anion Charge.
From www.youtube.com
KBr Lewis Structure How to Draw the Lewis Structure for KBr (potassium Bromide Anion Charge There is one cobalt ion. For example, iron(ii) has a 2+ charge; It is a conjugate base of a hydrogen. Bromide is a halide anion and a monoatomic bromine. This is because bromine has seven electrons in its outer shell, which. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. If we look at. Bromide Anion Charge.
From loeyfiwec.blob.core.windows.net
Chlorine Anion Symbol at Beverly Castleberry blog Bromide Anion Charge A bromide ion is an electrically charged atom of the element bromine. This is because bromine has seven electrons in its outer shell, which. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion. Bromide Anion Charge.
From utedzz.blogspot.com
Periodic Table With Common Ionic Charges Periodic Table Timeline Bromide Anion Charge A bromide ion is an electrically charged atom of the element bromine. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. There is one cobalt ion. Bromide is a halide anion and a monoatomic bromine. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. If we look. Bromide Anion Charge.
From www.youtube.com
Lewis Structure of CuBr2, copper (II) bromide YouTube Bromide Anion Charge For example, iron(ii) has a 2+ charge; Only one ion of each is needed to. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. Roman numeral notation indicates charge of ion when element commonly forms more than. Bromide Anion Charge.
From exoedcpam.blob.core.windows.net
Magnesium Bromide Ionic Bonding at Annie Hopson blog Bromide Anion Charge If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. This is because bromine has seven electrons in its outer shell, which. Only one ion of each is needed to. Predict the charge of monatomic main group elements. Bromide Anion Charge.
From www.numerade.com
SOLVED Ionic Compound Formulas For each of the ionic compounds below Bromide Anion Charge A bromide ion is an electrically charged atom of the element bromine. It is a conjugate base of a hydrogen. Bromide is a halide anion and a monoatomic bromine. Predict the charge of monatomic main group elements based on their group. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium. Bromide Anion Charge.
From www.numerade.com
SOLVED 1636 The benzene ring alters the reactivity of a neighboring Bromide Anion Charge There is one cobalt ion. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. This is because bromine has seven electrons in its outer shell, which. Predict the charge of monatomic main group elements based on their group. Bromide is a halide anion and a monoatomic bromine. A bromide ion is an electrically. Bromide Anion Charge.
From joimkpszm.blob.core.windows.net
Disadvantages Of Anion Pads at Javier Ward blog Bromide Anion Charge There is one cobalt ion. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. A bromide ion is an electrically charged atom of the element bromine. Roman numeral notation indicates charge of ion when element commonly forms. Bromide Anion Charge.
From www.fishersci.no
Antimony(III) bromide, 99.995 (metals basis), Thermo Scientific Bromide Anion Charge It is a conjugate base of a hydrogen. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. For example, iron(ii) has a 2+ charge; Only one ion of each is needed to. Predict the charge of monatomic. Bromide Anion Charge.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube Bromide Anion Charge Roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron(ii) has a 2+ charge; If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. Only one ion of each is. Bromide Anion Charge.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromide Anion Charge Predict the charge of monatomic main group elements based on their group. It is a conjugate base of a hydrogen. For example, iron(ii) has a 2+ charge; A bromide ion is an electrically charged atom of the element bromine. There is one cobalt ion. This is because bromine has seven electrons in its outer shell, which. Only one ion of. Bromide Anion Charge.
From www.numerade.com
SOLVED How does an atom of bromine79 a bromide ion with a 1 Bromide Anion Charge Roman numeral notation indicates charge of ion when element commonly forms more than one ion. There is one cobalt ion. This is because bromine has seven electrons in its outer shell, which. Predict the charge of monatomic main group elements based on their group. For example, iron(ii) has a 2+ charge; It is a conjugate base of a hydrogen. Bromide. Bromide Anion Charge.
From www.youtube.com
Lewis Structure of CsBr, caesium bromide YouTube Bromide Anion Charge There is one cobalt ion. It is a conjugate base of a hydrogen. A bromide ion is an electrically charged atom of the element bromine. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. Predict the charge. Bromide Anion Charge.
From www.numerade.com
SOLVEDFill in the blanks Formula Cation Anion Name CaF2 Bromide Anion Charge Only one ion of each is needed to. This is because bromine has seven electrons in its outer shell, which. Predict the charge of monatomic main group elements based on their group. For example, iron(ii) has a 2+ charge; There is one cobalt ion. A bromide ion is an electrically charged atom of the element bromine. Bromide is a halide. Bromide Anion Charge.
From www.youtube.com
How to Draw the Lewis Dot Structure for Br (Bromide ion) YouTube Bromide Anion Charge It is a conjugate base of a hydrogen. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. Bromide is a halide. Bromide Anion Charge.
From www.masterorganicchemistry.com
Benzylic Bromination and Benzylic Oxidation Master Organic Chemistry Bromide Anion Charge For example, iron(ii) has a 2+ charge; Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Bromide is a halide anion and a monoatomic bromine. There is one cobalt ion. This is because bromine has seven electrons in its outer shell, which. Roman numeral notation indicates charge of ion when element commonly forms. Bromide Anion Charge.
From www.dreamstime.com
Cations, Anions and Neutral Atom. Difference between Cation and Anion Bromide Anion Charge There is one cobalt ion. Predict the charge of monatomic main group elements based on their group. Only one ion of each is needed to. This is because bromine has seven electrons in its outer shell, which. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. Bromide is a halide anion and a monoatomic. Bromide Anion Charge.
From slideplayer.com
Unit 3 Nomenclature (Ch. 5) ppt download Bromide Anion Charge Only one ion of each is needed to. Predict the charge of monatomic main group elements based on their group. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. There is one cobalt ion. Bromide is a halide anion and a monoatomic bromine. If we look at the ionic compound consisting of lithium. Bromide Anion Charge.
From www.coursehero.com
[Solved] Cation Anion Formula Name sodium iodide aluminum bromide Bromide Anion Charge Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. There is one cobalt ion. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge. Bromide Anion Charge.
From www.numerade.com
SOLVED Texts File Edit View Insert Format Tools Addon 3 A 7 100 Bromide Anion Charge Bromide is a halide anion and a monoatomic bromine. Predict the charge of monatomic main group elements based on their group. Only one ion of each is needed to. There is one cobalt ion. A bromide ion is an electrically charged atom of the element bromine. Roman numeral notation indicates charge of ion when element commonly forms more than one. Bromide Anion Charge.
From ceukyrig.blob.core.windows.net
Aqueous Zinc Bromide Equation at Heather Spiers blog Bromide Anion Charge For example, iron(ii) has a 2+ charge; Predict the charge of monatomic main group elements based on their group. Bromide is a halide anion and a monoatomic bromine. This is because bromine has seven electrons in its outer shell, which. A bromide ion is an electrically charged atom of the element bromine. There is one cobalt ion. If we look. Bromide Anion Charge.
From slideplayer.com
Ionic charge and electrical conduction ppt download Bromide Anion Charge Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. It is a conjugate base of a hydrogen. This is because bromine has seven electrons in its outer shell, which. Only one ion of each is needed to. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. A. Bromide Anion Charge.
From www.masterorganicchemistry.com
What Makes A Good Nucleophile? Master Organic Chemistry Bromide Anion Charge If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. Bromide is a halide anion and a monoatomic bromine. For example, iron(ii) has a 2+ charge; Roman numeral notation indicates charge of ion when element commonly forms more. Bromide Anion Charge.
From pubs.acs.org
and Mechanism of Bromate−Bromide Reaction Catalyzed by Acetate Bromide Anion Charge Bromide is a halide anion and a monoatomic bromine. A bromide ion is an electrically charged atom of the element bromine. For example, iron(ii) has a 2+ charge; There is one cobalt ion. Predict the charge of monatomic main group elements based on their group. Only one ion of each is needed to. Recognize that metals lose electrons to form. Bromide Anion Charge.
From www.coursehero.com
[Solved] Construct a Lewis structure for potassium and bromine. Predict Bromide Anion Charge If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. It is a conjugate base of a hydrogen. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Bromide is a halide. Bromide Anion Charge.
From www.researchgate.net
Scheme 25. Bromide anion catalyzed alkylation of pyridinium salt Bromide Anion Charge For example, iron(ii) has a 2+ charge; Only one ion of each is needed to. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. It is a conjugate base of a hydrogen. A bromide ion is an electrically charged atom of the element bromine. This is because bromine has seven electrons in its. Bromide Anion Charge.