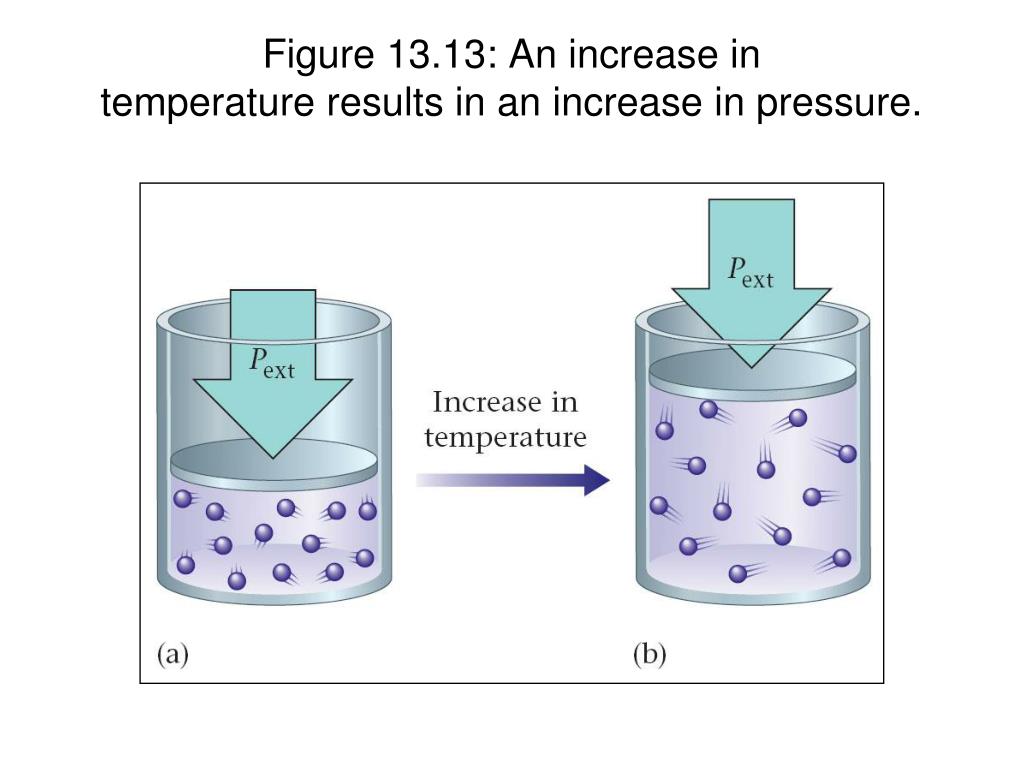
Does Pressure Increase As Temp . Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and. Yes, at constant density, the pressure increases as the temperature does: 1) by increasing the temperature which increases the average kinetic energy of the molecules i.e. You can increase pressure in two ways: Pressure increase with temperature calculator. Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant. In reality, most compression take place by reducing volume. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of.
from www.slideserve.com
You can increase pressure in two ways: 1) by increasing the temperature which increases the average kinetic energy of the molecules i.e. Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. In reality, most compression take place by reducing volume. For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. Pressure increase with temperature calculator. Yes, at constant density, the pressure increases as the temperature does: Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law.
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation
Does Pressure Increase As Temp If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Yes, at constant density, the pressure increases as the temperature does: Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. In reality, most compression take place by reducing volume. For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. 1) by increasing the temperature which increases the average kinetic energy of the molecules i.e. Pressure increase with temperature calculator. Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. You can increase pressure in two ways:
From www.youtube.com
The Effect of Temperature and Pressure on Solubility Mr C YouTube Does Pressure Increase As Temp Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and. Yes,. Does Pressure Increase As Temp.
From techiescientist.com
Does Density Change With Temperature? Techiescientist Does Pressure Increase As Temp In reality, most compression take place by reducing volume. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and. Yes, at constant density, the pressure increases as the temperature does: For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c. Does Pressure Increase As Temp.
From www.theweatherprediction.com
PRESSURE AND TEMPERATURE RELATIONSHIP Does Pressure Increase As Temp In reality, most compression take place by reducing volume. 1) by increasing the temperature which increases the average kinetic energy of the molecules i.e. Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. Yes, at constant density, the pressure increases as the temperature does: Pressure increase with temperature calculator. Early scientists explored the relationships among. Does Pressure Increase As Temp.
From socratic.org
How do mountains affect temperature on both the windward side and the Does Pressure Increase As Temp Yes, at constant density, the pressure increases as the temperature does: Pressure increase with temperature calculator. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v),. Does Pressure Increase As Temp.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an Does Pressure Increase As Temp If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. In reality, most compression take place by reducing volume. Pressure increase with temperature calculator. Pressure and temperature were fairly well understood in the age. Does Pressure Increase As Temp.
From jmpcoblog.com
Avoiding Pump Cavitation in Open Systems The Dynamic Relationship Does Pressure Increase As Temp In reality, most compression take place by reducing volume. You can increase pressure in two ways: Pressure increase with temperature calculator. Yes, at constant density, the pressure increases as the temperature does: Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables. Does Pressure Increase As Temp.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint Does Pressure Increase As Temp Pressure increase with temperature calculator. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant. You can increase pressure in two ways: Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. For example, having water sealed. Does Pressure Increase As Temp.
From www.pinterest.co.uk
A mathematical derivation of the equations relating the pressure Does Pressure Increase As Temp For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. You can increase pressure in two ways: If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. 1) by increasing the temperature which increases the average kinetic energy of. Does Pressure Increase As Temp.
From ar.inspiredpencil.com
Air Pressure Diagram For Kids Does Pressure Increase As Temp You can increase pressure in two ways: Yes, at constant density, the pressure increases as the temperature does: 1) by increasing the temperature which increases the average kinetic energy of the molecules i.e. For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. If you had a way to increase pressure. Does Pressure Increase As Temp.
From www.slideshare.net
Air pressure Does Pressure Increase As Temp Pressure increase with temperature calculator. Yes, at constant density, the pressure increases as the temperature does: For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. 1) by increasing. Does Pressure Increase As Temp.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Does Pressure Increase As Temp You can increase pressure in two ways: For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant. Yes, at constant density, the. Does Pressure Increase As Temp.
From klatordwr.blob.core.windows.net
Does Pressure Rise With Temperature at Eric Thomson blog Does Pressure Increase As Temp For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. Pressure increase with temperature calculator. Yes, at constant density, the pressure increases as the temperature does: Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. Early scientists explored the relationships among the pressure of a gas. Does Pressure Increase As Temp.
From klatordwr.blob.core.windows.net
Does Pressure Rise With Temperature at Eric Thomson blog Does Pressure Increase As Temp Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. Does Pressure Increase As Temp.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Does Pressure Increase As Temp For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there. Does Pressure Increase As Temp.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does Pressure Increase As Temp 1) by increasing the temperature which increases the average kinetic energy of the molecules i.e. Yes, at constant density, the pressure increases as the temperature does: Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and. For example, having water sealed at atmospheric pressure. Does Pressure Increase As Temp.
From klatordwr.blob.core.windows.net
Does Pressure Rise With Temperature at Eric Thomson blog Does Pressure Increase As Temp Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. Pressure increase with temperature calculator. Yes, at constant density, the pressure increases as the temperature does: 1) by increasing the temperature which increases the average kinetic energy of the molecules i.e. In reality, most compression take place by reducing volume. For example, having water sealed at. Does Pressure Increase As Temp.
From www.researchgate.net
1 The atmospheric density and pressure distribution. Download Does Pressure Increase As Temp You can increase pressure in two ways: 1) by increasing the temperature which increases the average kinetic energy of the molecules i.e. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and. If you had a way to increase pressure with no volume change,. Does Pressure Increase As Temp.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial Does Pressure Increase As Temp 1) by increasing the temperature which increases the average kinetic energy of the molecules i.e. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding. Does Pressure Increase As Temp.
From www.youtube.com
Equations of State part 1 understanding PressureVolume diagrams YouTube Does Pressure Increase As Temp Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant. In reality, most compression take place by reducing volume. 1) by increasing the temperature which increases the average kinetic energy of the molecules i.e. Pressure and temperature were fairly well understood. Does Pressure Increase As Temp.
From joifcnqsy.blob.core.windows.net
Does Partial Pressure Increase With Temperature at Linda Adams blog Does Pressure Increase As Temp Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. In reality, most compression take place by reducing volume. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and. You can increase pressure in two ways: Yes, at constant density,. Does Pressure Increase As Temp.
From socratic.org
A given mass of oxygen at room temperature occupies a volume of 500.0 Does Pressure Increase As Temp Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant. Pressure increase with temperature calculator. 1) by increasing the temperature which increases the average kinetic energy of the molecules i.e. In reality, most compression take place by reducing volume. Pressure and. Does Pressure Increase As Temp.
From techblog.ctgclean.com
Reducing Flow vs. Reducing Pressure Which is it? CTG Does Pressure Increase As Temp Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and. Pressure increase with temperature calculator.. Does Pressure Increase As Temp.
From engineerexcel.com
How Does Pressure Affect Melting Point? EngineerExcel Does Pressure Increase As Temp Yes, at constant density, the pressure increases as the temperature does: For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Early scientists explored the relationships among the pressure. Does Pressure Increase As Temp.
From docslib.org
Relationship Between Density, Pressure, and Temperature DocsLib Does Pressure Increase As Temp Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and. Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. You. Does Pressure Increase As Temp.
From www.youtube.com
Change in Temp and Pressure on Volumetric Flow Rates (example) YouTube Does Pressure Increase As Temp Pressure increase with temperature calculator. Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. Yes, at constant density, the pressure increases as the temperature does: If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Early scientists explored the relationships among the pressure. Does Pressure Increase As Temp.
From hidayaah.blob.core.windows.net
Unlocking The Mystery Exploring The Impact Of Temperature On Does Pressure Increase As Temp Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. 1) by increasing the temperature which increases the average kinetic energy of the molecules i.e. For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. Pressure and temperature were fairly well understood in the age of newton. Does Pressure Increase As Temp.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Does Pressure Increase As Temp Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and. For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. Pressure increase with temperature calculator. Initial pressure (p₁ in atm) initial temperature (t₁ in °c). Does Pressure Increase As Temp.
From courses.lumenlearning.com
Phase Changes Physics Does Pressure Increase As Temp 1) by increasing the temperature which increases the average kinetic energy of the molecules i.e. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Yes, at constant density, the pressure increases as the temperature does: Early scientists explored the relationships among the pressure of a gas (p). Does Pressure Increase As Temp.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Does Pressure Increase As Temp Yes, at constant density, the pressure increases as the temperature does: In reality, most compression take place by reducing volume. For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. Does Pressure Increase As Temp.
From cedric-bloggaines.blogspot.com
Describe How Atmospheric Pressure Changes as Altitude Increases. Does Pressure Increase As Temp If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. Yes, at constant density, the pressure increases as the temperature does: Pressure increase with temperature calculator. In reality, most compression take place by reducing. Does Pressure Increase As Temp.
From chem.libretexts.org
13.4 Effects of Temperature and Pressure on Solubility Chemistry Does Pressure Increase As Temp For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and. If you had a way to increase pressure with no volume change, then yes, temperature. Does Pressure Increase As Temp.
From masterconceptsinchemistry.com
As pressure increase the volume of gas decrease Does Pressure Increase As Temp For example, having water sealed at atmospheric pressure at 4c∘c 4 c ∘ c will have a density of. In reality, most compression take place by reducing volume. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Early scientists explored the relationships among the pressure of a. Does Pressure Increase As Temp.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Does Pressure Increase As Temp Pressure increase with temperature calculator. Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. Yes, at constant density, the pressure increases as the temperature does: You can increase pressure in two ways: Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding. Does Pressure Increase As Temp.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Does Pressure Increase As Temp 1) by increasing the temperature which increases the average kinetic energy of the molecules i.e. You can increase pressure in two ways: Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and. For example, having water sealed at atmospheric pressure at 4c∘c 4 c. Does Pressure Increase As Temp.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Does Pressure Increase As Temp In reality, most compression take place by reducing volume. 1) by increasing the temperature which increases the average kinetic energy of the molecules i.e. Initial pressure (p₁ in atm) initial temperature (t₁ in °c) final temperature (t₂ in. Yes, at constant density, the pressure increases as the temperature does: For example, having water sealed at atmospheric pressure at 4c∘c 4. Does Pressure Increase As Temp.