Atomic Mass Of Copper Is 63.54 Amu . The atomic masses of 63 cu and 65 cu are 62.9296 and 64.9278 amu respectively; The atomic mass of an element is the average mass of atoms of the element, measured in atomic mass units (amu). Average atomic mass = 63.62 amu. The atomic mass of copper is 63.54. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. Average atomic mass is the weighted average of the individual isotopes. If the atomic masses of 63cu and 65cu are 62.9296 and 64.9278 amu respectively what is the natural. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The given value of 63.54 amu is very close to this standard value, and the difference. The atomic mass of copper is 63.54. The atomic mass of copper is approximately 63.55 amu.
from www.numerade.com
The atomic mass of copper is 63.54. The atomic mass of copper is 63.54. If the atomic masses of 63cu and 65cu are 62.9296 and 64.9278 amu respectively what is the natural. Average atomic mass = 63.62 amu. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. Average atomic mass is the weighted average of the individual isotopes. The given value of 63.54 amu is very close to this standard value, and the difference. The atomic mass of an element is the average mass of atoms of the element, measured in atomic mass units (amu). Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. The atomic masses of 63 cu and 65 cu are 62.9296 and 64.9278 amu respectively;
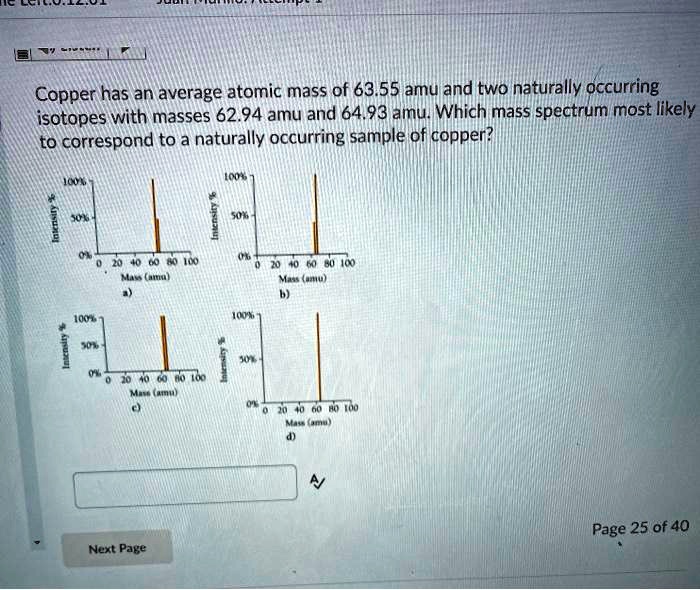
Copper has an average atomic mass of 63.55 amu and two naturally
Atomic Mass Of Copper Is 63.54 Amu The atomic mass of copper is approximately 63.55 amu. The given value of 63.54 amu is very close to this standard value, and the difference. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The atomic mass of an element is the average mass of atoms of the element, measured in atomic mass units (amu). Average atomic mass = 63.62 amu. If the atomic masses of 63cu and 65cu are 62.9296 and 64.9278 amu respectively what is the natural. Average atomic mass is the weighted average of the individual isotopes. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. The atomic mass of copper is 63.54. The atomic masses of 63 cu and 65 cu are 62.9296 and 64.9278 amu respectively; The atomic mass of copper is 63.54. The atomic mass of copper is approximately 63.55 amu.
From www.wikihow.com
3 Clear and Easy Ways to Calculate Atomic Mass wikiHow Atomic Mass Of Copper Is 63.54 Amu The atomic masses of 63 cu and 65 cu are 62.9296 and 64.9278 amu respectively; The atomic mass of copper is 63.54. The given value of 63.54 amu is very close to this standard value, and the difference. The atomic mass of copper is 63.54. The atomic mass of copper is approximately 63.55 amu. Average atomic mass is the weighted. Atomic Mass Of Copper Is 63.54 Amu.
From www.numerade.com
SOLVED calculate the mass in grams for each of the following 0.75 mole Atomic Mass Of Copper Is 63.54 Amu The atomic mass of copper is approximately 63.55 amu. The atomic mass of copper is 63.54. Average atomic mass = 63.62 amu. The atomic mass of copper is 63.54. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average. Atomic Mass Of Copper Is 63.54 Amu.
From www.numerade.com
SOLVED The atomic mass of copper metal is 63.6 amu calculate the mass Atomic Mass Of Copper Is 63.54 Amu This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. The atomic. Atomic Mass Of Copper Is 63.54 Amu.
From www.alamy.com
Cu Copper Chemical Element Periodic Table. Single vector illustration Atomic Mass Of Copper Is 63.54 Amu The atomic mass of an element is the average mass of atoms of the element, measured in atomic mass units (amu). Average atomic mass is the weighted average of the individual isotopes. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting. Atomic Mass Of Copper Is 63.54 Amu.
From www.chegg.com
Solved 1. Which of the following are isotopes of the same Atomic Mass Of Copper Is 63.54 Amu If the atomic masses of 63cu and 65cu are 62.9296 and 64.9278 amu respectively what is the natural. The atomic mass of copper is 63.54. The atomic mass of an element is the average mass of atoms of the element, measured in atomic mass units (amu). The atomic mass of copper is 63.54. Average atomic mass is the weighted average. Atomic Mass Of Copper Is 63.54 Amu.
From www.numerade.com
SOLVEDObtain the fractional abundances for the two naturally occurring Atomic Mass Of Copper Is 63.54 Amu The atomic mass of copper is approximately 63.55 amu. The atomic mass of copper is 63.54. The atomic mass of an element is the average mass of atoms of the element, measured in atomic mass units (amu). The atomic mass of copper is 63.54. Average atomic mass = 63.62 amu. The atomic masses of 63 cu and 65 cu are. Atomic Mass Of Copper Is 63.54 Amu.
From askfilo.com
What mass of copper will contain 3.22×1024 atoms of copper? (Atomic mass Atomic Mass Of Copper Is 63.54 Amu Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. The atomic mass of copper is 63.54. Average atomic mass is the weighted average of the individual isotopes. If the atomic masses of 63cu and 65cu are 62.9296. Atomic Mass Of Copper Is 63.54 Amu.
From www.chegg.com
Solved Copper has an atomic mass of 63.55 amu and two Atomic Mass Of Copper Is 63.54 Amu The atomic mass of copper is approximately 63.55 amu. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. The given value of 63.54 amu is very close to this standard value, and the difference. This atomic mass. Atomic Mass Of Copper Is 63.54 Amu.
From www.vrogue.co
The Average Atomic Mass Of Copper Is 63 546 Amu Natur vrogue.co Atomic Mass Of Copper Is 63.54 Amu Average atomic mass = 63.62 amu. The given value of 63.54 amu is very close to this standard value, and the difference. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The atomic mass of an element is the average mass of atoms. Atomic Mass Of Copper Is 63.54 Amu.
From www.numerade.com
SOLVEDWhat is the mass in grams of one copper atom? Atomic Mass Of Copper Is 63.54 Amu Average atomic mass is the weighted average of the individual isotopes. The atomic mass of copper is approximately 63.55 amu. The atomic mass of copper is 63.54. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The atomic masses of 63 cu and. Atomic Mass Of Copper Is 63.54 Amu.
From inspiritvr.com
Atomic Mass Unit Study Guide Inspirit Atomic Mass Of Copper Is 63.54 Amu Average atomic mass is the weighted average of the individual isotopes. The given value of 63.54 amu is very close to this standard value, and the difference. The atomic mass of copper is approximately 63.55 amu. The atomic mass of an element is the average mass of atoms of the element, measured in atomic mass units (amu). Copper has two. Atomic Mass Of Copper Is 63.54 Amu.
From www.numerade.com
SOLVED A copper wire having a radius of 2.00 mm carries a current of 5 Atomic Mass Of Copper Is 63.54 Amu The atomic mass of copper is 63.54. The given value of 63.54 amu is very close to this standard value, and the difference. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. Average atomic mass is the. Atomic Mass Of Copper Is 63.54 Amu.
From itechguide.pages.dev
Is Copper An Element itechguide Atomic Mass Of Copper Is 63.54 Amu The atomic mass of copper is 63.54. The atomic mass of copper is approximately 63.55 amu. The atomic mass of copper is 63.54. Average atomic mass = 63.62 amu. The atomic mass of an element is the average mass of atoms of the element, measured in atomic mass units (amu). Average atomic mass is the weighted average of the individual. Atomic Mass Of Copper Is 63.54 Amu.
From www.vrogue.co
The Average Atomic Mass Of Copper Is 63 546 Amu Natur vrogue.co Atomic Mass Of Copper Is 63.54 Amu Average atomic mass is the weighted average of the individual isotopes. If the atomic masses of 63cu and 65cu are 62.9296 and 64.9278 amu respectively what is the natural. The atomic masses of 63 cu and 65 cu are 62.9296 and 64.9278 amu respectively; The atomic mass of copper is approximately 63.55 amu. Copper has two isotopes, 63 cu (69.15%,. Atomic Mass Of Copper Is 63.54 Amu.
From www.animalia-life.club
Modern Periodic Table With Atomic Mass And Atomic Number Atomic Mass Of Copper Is 63.54 Amu The atomic mass of copper is approximately 63.55 amu. The atomic mass of copper is 63.54. If the atomic masses of 63cu and 65cu are 62.9296 and 64.9278 amu respectively what is the natural. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and. Atomic Mass Of Copper Is 63.54 Amu.
From www.toppr.com
6. The density of copper is 9 x 103 kg/m and its atomic mass is 63.5 u Atomic Mass Of Copper Is 63.54 Amu The given value of 63.54 amu is very close to this standard value, and the difference. The atomic mass of copper is 63.54. The atomic mass of an element is the average mass of atoms of the element, measured in atomic mass units (amu). The atomic mass of copper is 63.54. If the atomic masses of 63cu and 65cu are. Atomic Mass Of Copper Is 63.54 Amu.
From www.numerade.com
SOLVED Calculate Copper has two isotopes C u63 (abundance =69.2 Atomic Mass Of Copper Is 63.54 Amu Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. The given value of 63.54 amu is very close to this standard value, and the difference. This atomic mass calculator shows you how to find the atomic mass. Atomic Mass Of Copper Is 63.54 Amu.
From askfilo.com
Copper has two naturally occurring isotopes 63Cu and 65Cu. If the average.. Atomic Mass Of Copper Is 63.54 Amu The atomic mass of copper is 63.54. Average atomic mass = 63.62 amu. The atomic mass of copper is approximately 63.55 amu. Average atomic mass is the weighted average of the individual isotopes. The given value of 63.54 amu is very close to this standard value, and the difference. The atomic masses of 63 cu and 65 cu are 62.9296. Atomic Mass Of Copper Is 63.54 Amu.
From www.nuclear-power.com
Iron Atomic Number Atomic Mass Density of Iron Atomic Mass Of Copper Is 63.54 Amu The atomic mass of copper is approximately 63.55 amu. The atomic mass of an element is the average mass of atoms of the element, measured in atomic mass units (amu). This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The given value of. Atomic Mass Of Copper Is 63.54 Amu.
From www.slideserve.com
PPT What is atomic mass? PowerPoint Presentation, free download ID Atomic Mass Of Copper Is 63.54 Amu This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. Average atomic. Atomic Mass Of Copper Is 63.54 Amu.
From askfilo.com
What mass of copper will contain 3.22×1024 atoms of copper?(Atomic mass Atomic Mass Of Copper Is 63.54 Amu The given value of 63.54 amu is very close to this standard value, and the difference. The atomic mass of copper is 63.54. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The atomic masses of 63 cu and 65 cu are 62.9296. Atomic Mass Of Copper Is 63.54 Amu.
From material-properties.org
Copper Periodic Table and Atomic Properties Atomic Mass Of Copper Is 63.54 Amu The given value of 63.54 amu is very close to this standard value, and the difference. Average atomic mass is the weighted average of the individual isotopes. If the atomic masses of 63cu and 65cu are 62.9296 and 64.9278 amu respectively what is the natural. The atomic masses of 63 cu and 65 cu are 62.9296 and 64.9278 amu respectively;. Atomic Mass Of Copper Is 63.54 Amu.
From www.expii.com
Atomic Mass — Definition & AMU Expii Atomic Mass Of Copper Is 63.54 Amu Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. The atomic masses of 63 cu and 65 cu are 62.9296 and 64.9278 amu respectively; The atomic mass of an element is the average mass of atoms of. Atomic Mass Of Copper Is 63.54 Amu.
From ecurrencythailand.com
What Is The Atomic Mass Of Copper 63 And Copper 65? All Answers Atomic Mass Of Copper Is 63.54 Amu The given value of 63.54 amu is very close to this standard value, and the difference. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. Average atomic mass is the weighted average of the individual isotopes. The. Atomic Mass Of Copper Is 63.54 Amu.
From www.numerade.com
SOLVED Copper has two isotopes, Cu63 and Cu65. The table below shows Atomic Mass Of Copper Is 63.54 Amu The given value of 63.54 amu is very close to this standard value, and the difference. The atomic mass of copper is approximately 63.55 amu. Average atomic mass is the weighted average of the individual isotopes. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic. Atomic Mass Of Copper Is 63.54 Amu.
From brainly.ph
Determine the number of atoms in 2g copper where atomic mass of copper Atomic Mass Of Copper Is 63.54 Amu Average atomic mass = 63.62 amu. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The atomic mass of an element is the average mass of atoms of the element, measured in atomic mass units (amu). If the atomic masses of 63cu and. Atomic Mass Of Copper Is 63.54 Amu.
From www.pinterest.co.uk
Copper, atomic structure Stock Image C046/0322 Atomic structure Atomic Mass Of Copper Is 63.54 Amu Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. The atomic mass of copper is 63.54. If the atomic masses of 63cu and 65cu are 62.9296 and 64.9278 amu respectively what is the natural. Average atomic mass. Atomic Mass Of Copper Is 63.54 Amu.
From www.numerade.com
Calculate the relative atomic mass of copper with the following Atomic Mass Of Copper Is 63.54 Amu If the atomic masses of 63cu and 65cu are 62.9296 and 64.9278 amu respectively what is the natural. The atomic mass of copper is approximately 63.55 amu. The atomic mass of copper is 63.54. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and. Atomic Mass Of Copper Is 63.54 Amu.
From www.teachoo.com
Definition, How to caluclate Atomic mass Teachoo Science Atomic Mass Of Copper Is 63.54 Amu Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. The atomic mass of copper is 63.54. The given value of 63.54 amu is very close to this standard value, and the difference. Average atomic mass = 63.62. Atomic Mass Of Copper Is 63.54 Amu.
From eliottrebekah.blogspot.com
8+ Average Atomic Mass Calculator EliottRebekah Atomic Mass Of Copper Is 63.54 Amu Average atomic mass = 63.62 amu. If the atomic masses of 63cu and 65cu are 62.9296 and 64.9278 amu respectively what is the natural. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The atomic mass of copper is 63.54. The atomic masses. Atomic Mass Of Copper Is 63.54 Amu.
From www.vrogue.co
The Average Atomic Mass Of Copper Is 63 546 Amu Natur vrogue.co Atomic Mass Of Copper Is 63.54 Amu This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The atomic masses of 63 cu and 65 cu are 62.9296 and 64.9278 amu respectively; Average atomic mass is the weighted average of the individual isotopes. Copper has two isotopes, 63 cu (69.15%, mass=62.9300. Atomic Mass Of Copper Is 63.54 Amu.
From www.numerade.com
Copper has an average atomic mass of 63.55 amu and two naturally Atomic Mass Of Copper Is 63.54 Amu The atomic masses of 63 cu and 65 cu are 62.9296 and 64.9278 amu respectively; The atomic mass of copper is 63.54. Average atomic mass = 63.62 amu. If the atomic masses of 63cu and 65cu are 62.9296 and 64.9278 amu respectively what is the natural. This atomic mass calculator shows you how to find the atomic mass of an. Atomic Mass Of Copper Is 63.54 Amu.
From kunduz.com
[ANSWERED] The density of copper is 9 10 kg m and its atomic mass is 63 Atomic Mass Of Copper Is 63.54 Amu The atomic mass of copper is approximately 63.55 amu. The given value of 63.54 amu is very close to this standard value, and the difference. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. If the atomic. Atomic Mass Of Copper Is 63.54 Amu.
From quizlet.com
Using the periodic table, determine the atomic mass of coppe Quizlet Atomic Mass Of Copper Is 63.54 Amu The atomic masses of 63 cu and 65 cu are 62.9296 and 64.9278 amu respectively; The atomic mass of an element is the average mass of atoms of the element, measured in atomic mass units (amu). Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are. Atomic Mass Of Copper Is 63.54 Amu.
From www.vectorstock.com
Symbol and electron diagram for copper Royalty Free Vector Atomic Mass Of Copper Is 63.54 Amu Average atomic mass = 63.62 amu. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. Average atomic mass is the weighted average of the individual isotopes. If the atomic masses of 63cu and 65cu are 62.9296 and 64.9278 amu respectively what is the. Atomic Mass Of Copper Is 63.54 Amu.