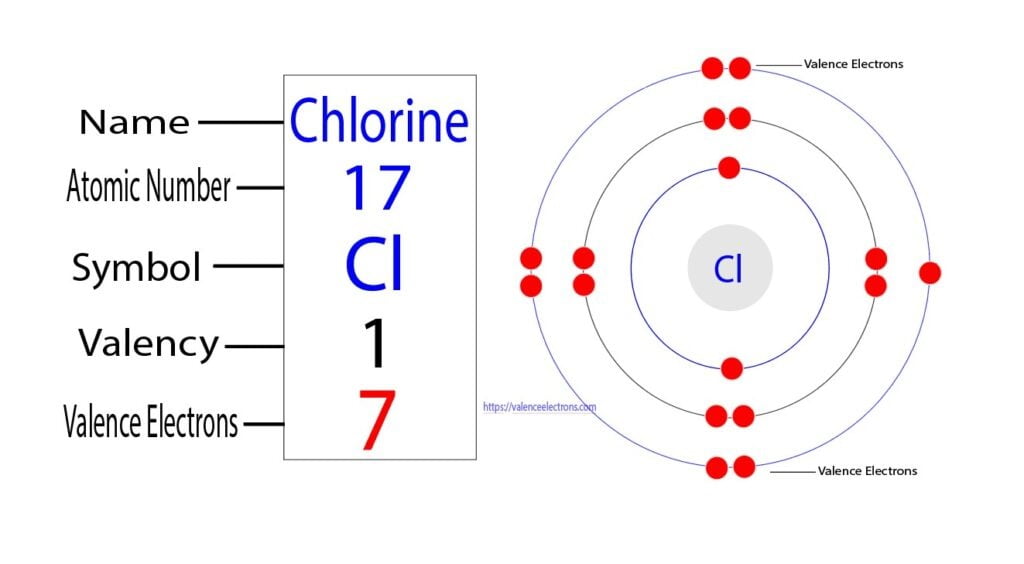
Chlorine Electron De Valence . It describes how electrons are distributed among the various. Valence electrons are the number of electrons present in the outermost shell of an atom. The electrons will be placed in different orbitals according to the energy level:. The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. Chlorine has an electron configuration of 1s22s22p6,3s23p5. 119 rows valence electrons: Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. The valence electron configuration for chlorine is s2p5. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. The last shell of a chlorine atom has 7.
from valenceelectrons.com
The electrons will be placed in different orbitals according to the energy level:. The last shell of a chlorine atom has 7. Valence electrons are the number of electrons present in the outermost shell of an atom. 119 rows valence electrons: The shorthand electron configuration (or noble gas configuration) as well as. Chlorine has an electron configuration of 1s22s22p6,3s23p5. The valence electron configuration for chlorine is s2p5. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. It describes how electrons are distributed among the various. The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals.
How Many Valence Electrons Does Aluminum Chloride Have?
Chlorine Electron De Valence The last shell of a chlorine atom has 7. The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. The shorthand electron configuration (or noble gas configuration) as well as. Chlorine has an electron configuration of 1s22s22p6,3s23p5. Electron configuration chart of all elements is mentioned in the table below. The valence electron configuration for chlorine is s2p5. It describes how electrons are distributed among the various. Valence electrons are the number of electrons present in the outermost shell of an atom. 119 rows valence electrons: The last shell of a chlorine atom has 7. The electrons will be placed in different orbitals according to the energy level:. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals.
From gioisrptn.blob.core.windows.net
Cl Electron Structure at Nicole Sellars blog Chlorine Electron De Valence The last shell of a chlorine atom has 7. The valence electron configuration for chlorine is s2p5. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. Chlorine has an electron configuration of 1s22s22p6,3s23p5. The electron configuration of chlorine refers to the arrangement of electrons in the. Chlorine Electron De Valence.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Electron De Valence The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. The electrons will be placed in different orbitals according to the energy level:. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. The last shell of a chlorine. Chlorine Electron De Valence.
From byjus.com
In the electron dot structure, the valence shell electrons are Chlorine Electron De Valence The electrons will be placed in different orbitals according to the energy level:. Valence electrons are the number of electrons present in the outermost shell of an atom. The valence electron configuration for chlorine is s2p5. Chlorine has an electron configuration of 1s22s22p6,3s23p5. It describes how electrons are distributed among the various. The last shell of a chlorine atom has. Chlorine Electron De Valence.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Electron De Valence Chlorine has an electron configuration of 1s22s22p6,3s23p5. The last shell of a chlorine atom has 7. Electron configuration chart of all elements is mentioned in the table below. It describes how electrons are distributed among the various. 119 rows valence electrons: Valence electrons are the number of electrons present in the outermost shell of an atom. The shorthand electron configuration. Chlorine Electron De Valence.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Electron De Valence The electrons will be placed in different orbitals according to the energy level:. 119 rows valence electrons: Valence electrons are the number of electrons present in the outermost shell of an atom. Chlorine has an electron configuration of 1s22s22p6,3s23p5. The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. The last shell. Chlorine Electron De Valence.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence Chlorine Electron De Valence The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. It describes how electrons are distributed among the various. The last shell of a chlorine atom has 7. The valence electron configuration for chlorine is s2p5. 119 rows valence electrons: Electron configuration chart of all elements is mentioned in the table below.. Chlorine Electron De Valence.
From www.chegg.com
Solved Calcium has two valence electrons and an Chlorine Electron De Valence The electrons will be placed in different orbitals according to the energy level:. The valence electron configuration for chlorine is s2p5. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. Valence electrons are the number of electrons present in the outermost shell of an atom. Electron configuration chart of all elements is mentioned. Chlorine Electron De Valence.
From guide-scientific.com
¿Cuántos electrones de valencia tiene el Cloro (Cl)? Valencia de cloro. Chlorine Electron De Valence The valence electron configuration for chlorine is s2p5. It describes how electrons are distributed among the various. The shorthand electron configuration (or noble gas configuration) as well as. The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. 119 rows valence electrons: The electrons will be placed in different orbitals according to. Chlorine Electron De Valence.
From www.shutterstock.com
16 Bohr Model Chlorine Images, Stock Photos & Vectors Shutterstock Chlorine Electron De Valence It describes how electrons are distributed among the various. Chlorine has an electron configuration of 1s22s22p6,3s23p5. Electron configuration chart of all elements is mentioned in the table below. The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. The shorthand electron configuration (or noble gas configuration) as well as. The last shell. Chlorine Electron De Valence.
From material-properties.org
Sulfur and Chlorine Comparison Properties Material Properties Chlorine Electron De Valence The last shell of a chlorine atom has 7. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. The valence electron configuration for chlorine is s2p5. 119 rows valence electrons: The electrons will be placed in different orbitals according to the energy level:. The chlorine atom has a total of 17 electrons so,. Chlorine Electron De Valence.
From favpng.com
Atom Bohr Model Electron Configuration Chlorine, PNG, 1000x1000px, Atom Chlorine Electron De Valence The shorthand electron configuration (or noble gas configuration) as well as. 119 rows valence electrons: The last shell of a chlorine atom has 7. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. Valence electrons are the number of electrons present in the outermost shell of an atom. It describes how electrons are. Chlorine Electron De Valence.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Chlorine Electron De Valence Valence electrons are the number of electrons present in the outermost shell of an atom. Chlorine has an electron configuration of 1s22s22p6,3s23p5. The electrons will be placed in different orbitals according to the energy level:. It describes how electrons are distributed among the various. 119 rows valence electrons: The chlorine atom has a total of 17 electrons so, we have. Chlorine Electron De Valence.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition, electron Chlorine Electron De Valence Valence electrons are the number of electrons present in the outermost shell of an atom. Electron configuration chart of all elements is mentioned in the table below. It describes how electrons are distributed among the various. Chlorine has an electron configuration of 1s22s22p6,3s23p5. The electrons will be placed in different orbitals according to the energy level:. The last shell of. Chlorine Electron De Valence.
From valenceelectrons.com
How Many Valence Electrons Does Aluminum Chloride Have? Chlorine Electron De Valence The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. The shorthand electron configuration (or noble gas configuration) as well as. It describes how electrons are distributed among the various. Electron configuration chart of all elements is mentioned in the table below. 119 rows valence electrons: The last shell of a chlorine. Chlorine Electron De Valence.
From www.transtutors.com
(Get Answer) What is the electron configuration for Cl, chlorine, and Chlorine Electron De Valence The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. The last shell of a chlorine atom has 7. Valence electrons are the number of electrons present in the outermost shell of an atom. It describes how electrons are distributed among the various. 119 rows valence electrons: The electrons will be placed. Chlorine Electron De Valence.
From imgbin.com
Electron Configuration Aufbau Principle Valence Electron Chlorine PNG Chlorine Electron De Valence The electrons will be placed in different orbitals according to the energy level:. 119 rows valence electrons: The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. The last shell of a chlorine atom has 7. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration. Chlorine Electron De Valence.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Electron De Valence 119 rows valence electrons: Electron configuration chart of all elements is mentioned in the table below. The last shell of a chlorine atom has 7. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. The shorthand electron configuration (or noble gas configuration) as well as. Valence electrons are the number of electrons present. Chlorine Electron De Valence.
From chamotgallery.com
How many protons, neutrons and electrons does chlorine have? (2023) Chlorine Electron De Valence The shorthand electron configuration (or noble gas configuration) as well as. The electrons will be placed in different orbitals according to the energy level:. Valence electrons are the number of electrons present in the outermost shell of an atom. Electron configuration chart of all elements is mentioned in the table below. It describes how electrons are distributed among the various.. Chlorine Electron De Valence.
From valenceelectrons.com
How many valence electrons does carbon(C) have? Chlorine Electron De Valence It describes how electrons are distributed among the various. Valence electrons are the number of electrons present in the outermost shell of an atom. The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. Chlorine has an electron configuration of 1s22s22p6,3s23p5. The shorthand electron configuration (or noble gas configuration) as well as.. Chlorine Electron De Valence.
From pixels.com
Chlorine Electron Configuration Photograph by Chlorine Electron De Valence The last shell of a chlorine atom has 7. The electrons will be placed in different orbitals according to the energy level:. The valence electron configuration for chlorine is s2p5. The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. 119 rows valence electrons: Electron configuration chart of all elements is mentioned. Chlorine Electron De Valence.
From www.dreamstime.com
Chlorine stock illustration. Illustration of isolated 83614864 Chlorine Electron De Valence The shorthand electron configuration (or noble gas configuration) as well as. The electrons will be placed in different orbitals according to the energy level:. The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. Valence electrons are the number of electrons present in the outermost shell of an atom. The electron configuration. Chlorine Electron De Valence.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Electron De Valence 119 rows valence electrons: It describes how electrons are distributed among the various. Valence electrons are the number of electrons present in the outermost shell of an atom. The valence electron configuration for chlorine is s2p5. The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. The electron configuration of chlorine refers. Chlorine Electron De Valence.
From www.youtube.com
How to Draw the Lewis Structure for ClO2 (Chlorine dioxide) YouTube Chlorine Electron De Valence The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. The valence electron configuration for chlorine is s2p5. The last shell of a chlorine atom has 7. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. Electron configuration chart of all elements is mentioned in. Chlorine Electron De Valence.
From www.klipartz.com
Lewis structure Chlorine Valence electron Diagram, 18, miscellaneous Chlorine Electron De Valence 119 rows valence electrons: The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. The electrons will be placed in different orbitals according to the energy level:. Electron configuration chart of all elements is mentioned in the table below. The last shell of a chlorine atom has 7. Chlorine has an electron. Chlorine Electron De Valence.
From drivenheisenberg.blogspot.com
Electron Dot Diagram For Chlorine Drivenheisenberg Chlorine Electron De Valence The valence electron configuration for chlorine is s2p5. Chlorine has an electron configuration of 1s22s22p6,3s23p5. 119 rows valence electrons: The electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. The last shell of a chlorine atom. Chlorine Electron De Valence.
From favpng.com
Lewis Structure Chlorine Valence Electron Diagram, PNG, 2000x2000px Chlorine Electron De Valence Chlorine has an electron configuration of 1s22s22p6,3s23p5. The shorthand electron configuration (or noble gas configuration) as well as. It describes how electrons are distributed among the various. Valence electrons are the number of electrons present in the outermost shell of an atom. The last shell of a chlorine atom has 7. 119 rows valence electrons: The electron configuration of chlorine. Chlorine Electron De Valence.
From valenceelectrons.com
How Many Valence Electrons Does COCl2 (phosgene) Have? Chlorine Electron De Valence It describes how electrons are distributed among the various. Valence electrons are the number of electrons present in the outermost shell of an atom. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. The shorthand electron configuration (or noble gas configuration) as well as. The valence electron configuration for chlorine is s2p5. Chlorine. Chlorine Electron De Valence.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine Trifluoride Have? Chlorine Electron De Valence The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. The electrons will be placed in different orbitals according to the energy level:. The shorthand electron configuration (or noble gas configuration) as well as. It describes how electrons are distributed among the various. The electron configuration of chlorine refers to the arrangement. Chlorine Electron De Valence.
From www.shutterstock.com
3.722 Atom structure of chlorine Görseli, Stok Fotoğraflar ve Vektörler Chlorine Electron De Valence 119 rows valence electrons: The last shell of a chlorine atom has 7. Valence electrons are the number of electrons present in the outermost shell of an atom. Chlorine has an electron configuration of 1s22s22p6,3s23p5. The electrons will be placed in different orbitals according to the energy level:. Electron configuration chart of all elements is mentioned in the table below.. Chlorine Electron De Valence.
From www.ck12.org
Valence Electrons CK12 Foundation Chlorine Electron De Valence The valence electron configuration for chlorine is s2p5. Valence electrons are the number of electrons present in the outermost shell of an atom. The last shell of a chlorine atom has 7. The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. It describes how electrons are distributed among the various. The. Chlorine Electron De Valence.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Electron De Valence The shorthand electron configuration (or noble gas configuration) as well as. The valence electron configuration for chlorine is s2p5. Chlorine has an electron configuration of 1s22s22p6,3s23p5. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. The chlorine atom has a total. Chlorine Electron De Valence.
From www.slideserve.com
PPT Electron Energy Levels and Configurations PowerPoint Presentation Chlorine Electron De Valence The last shell of a chlorine atom has 7. Chlorine has an electron configuration of 1s22s22p6,3s23p5. The electrons will be placed in different orbitals according to the energy level:. The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. The shorthand electron configuration (or noble gas configuration) as well as. It describes. Chlorine Electron De Valence.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Chlorine Electron De Valence The last shell of a chlorine atom has 7. Valence electrons are the number of electrons present in the outermost shell of an atom. 119 rows valence electrons: The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. The electrons will be placed in different orbitals according to the energy level:. The. Chlorine Electron De Valence.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Chlorine Electron De Valence The shorthand electron configuration (or noble gas configuration) as well as. Valence electrons are the number of electrons present in the outermost shell of an atom. It describes how electrons are distributed among the various. Electron configuration chart of all elements is mentioned in the table below. The last shell of a chlorine atom has 7. The electron configuration of. Chlorine Electron De Valence.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Electron De Valence 119 rows valence electrons: The electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. The valence electron configuration for chlorine is s2p5. The chlorine atom has a total of 17 electrons so, we have to put 17 electrons in orbitals. Electron configuration chart of all elements is mentioned in the table below. It describes. Chlorine Electron De Valence.