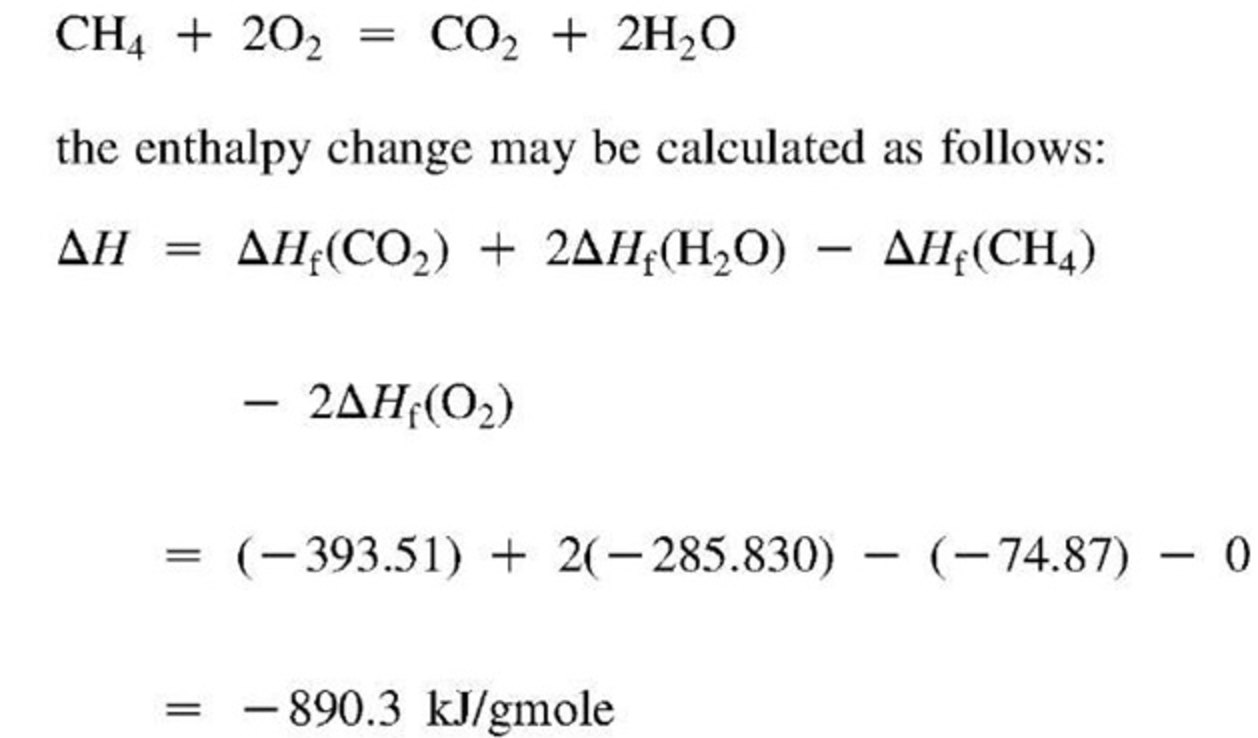Standard Enthalpy Of Formation Is Zero For Zno . The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. For an element the standard state is the form in which the element exists (is more stable) under condition of 1 bar and at the temperature of. Most values of standard enthalpy of formation are negative, indicating that these compounds are formed through exothermic reactions. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Characteristics of enthalpy of formation. The standard enthalpy of formation of any element in its most stable form is zero by definition. Why is that a convention? 193 rows the change in enthalpy for a reaction can be calculated from the enthalpies of formation of the reactants and the products. The enthalpy difference between graphite and diamond is too large for both to have a standard enthalpy of formation of zero. The standard enthalpy of formation of any element in its standard state is.
from storage.googleapis.com
Characteristics of enthalpy of formation. 193 rows the change in enthalpy for a reaction can be calculated from the enthalpies of formation of the reactants and the products. Why is that a convention? Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element in its most stable form is zero by definition. Most values of standard enthalpy of formation are negative, indicating that these compounds are formed through exothermic reactions. The standard enthalpy of formation of any element in its standard state is. For an element the standard state is the form in which the element exists (is more stable) under condition of 1 bar and at the temperature of. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. The enthalpy difference between graphite and diamond is too large for both to have a standard enthalpy of formation of zero.
Standard Enthalpy Of Formation Sulfuric Acid
Standard Enthalpy Of Formation Is Zero For Zno The standard enthalpy of formation of any element in its most stable form is zero by definition. Why is that a convention? Most values of standard enthalpy of formation are negative, indicating that these compounds are formed through exothermic reactions. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. Characteristics of enthalpy of formation. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element in its standard state is. The enthalpy difference between graphite and diamond is too large for both to have a standard enthalpy of formation of zero. For an element the standard state is the form in which the element exists (is more stable) under condition of 1 bar and at the temperature of. 193 rows the change in enthalpy for a reaction can be calculated from the enthalpies of formation of the reactants and the products. The standard enthalpy of formation of any element in its most stable form is zero by definition.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5773812 Standard Enthalpy Of Formation Is Zero For Zno Why is that a convention? Characteristics of enthalpy of formation. The standard enthalpy of formation of any element in its most stable form is zero by definition. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of. Standard Enthalpy Of Formation Is Zero For Zno.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Is Zero For Zno The standard enthalpy of formation of any element in its standard state is. The enthalpy difference between graphite and diamond is too large for both to have a standard enthalpy of formation of zero. For an element the standard state is the form in which the element exists (is more stable) under condition of 1 bar and at the temperature. Standard Enthalpy Of Formation Is Zero For Zno.
From askfilo.com
Consider the following. (a) Standard enthalpy of formation of Br2 (I) is Standard Enthalpy Of Formation Is Zero For Zno Characteristics of enthalpy of formation. Most values of standard enthalpy of formation are negative, indicating that these compounds are formed through exothermic reactions. The standard enthalpy of formation of any element in its standard state is. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. The standard enthalpy of formation of any element in its. Standard Enthalpy Of Formation Is Zero For Zno.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Is Zero For Zno The standard enthalpy of formation of any element in its most stable form is zero by definition. The enthalpy difference between graphite and diamond is too large for both to have a standard enthalpy of formation of zero. Most values of standard enthalpy of formation are negative, indicating that these compounds are formed through exothermic reactions. Standard enthalpy change of. Standard Enthalpy Of Formation Is Zero For Zno.
From askfilo.com
From the given information, what is the standard enthalpy of formation fo.. Standard Enthalpy Of Formation Is Zero For Zno Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Characteristics of enthalpy of formation. Most values of standard enthalpy of formation are negative, indicating that these compounds are formed through exothermic reactions. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. For an. Standard Enthalpy Of Formation Is Zero For Zno.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Is Zero For Zno Characteristics of enthalpy of formation. Most values of standard enthalpy of formation are negative, indicating that these compounds are formed through exothermic reactions. The enthalpy difference between graphite and diamond is too large for both to have a standard enthalpy of formation of zero. 193 rows the change in enthalpy for a reaction can be calculated from the enthalpies of. Standard Enthalpy Of Formation Is Zero For Zno.
From www.numerade.com
SOLVED A scientist measures the standard enthalpy change for the Standard Enthalpy Of Formation Is Zero For Zno Characteristics of enthalpy of formation. Why is that a convention? The enthalpy difference between graphite and diamond is too large for both to have a standard enthalpy of formation of zero. 193 rows the change in enthalpy for a reaction can be calculated from the enthalpies of formation of the reactants and the products. The standard enthalpy of formation of. Standard Enthalpy Of Formation Is Zero For Zno.
From www.chegg.com
Solved The standard enthalpy of formation (ΔHf∘) is the Standard Enthalpy Of Formation Is Zero For Zno For an element the standard state is the form in which the element exists (is more stable) under condition of 1 bar and at the temperature of. Most values of standard enthalpy of formation are negative, indicating that these compounds are formed through exothermic reactions. The enthalpy difference between graphite and diamond is too large for both to have a. Standard Enthalpy Of Formation Is Zero For Zno.
From www.studocu.com
Standard Enthalpy OF Formation C 2 H 2 (g) +226 H 2 SO 4 (l) −811 Standard Enthalpy Of Formation Is Zero For Zno For an element the standard state is the form in which the element exists (is more stable) under condition of 1 bar and at the temperature of. Why is that a convention? 193 rows the change in enthalpy for a reaction can be calculated from the enthalpies of formation of the reactants and the products. The standard enthalpy of formation. Standard Enthalpy Of Formation Is Zero For Zno.
From people.chem.umass.edu
to Adobe GoLive 6 Standard Enthalpy Of Formation Is Zero For Zno Characteristics of enthalpy of formation. Most values of standard enthalpy of formation are negative, indicating that these compounds are formed through exothermic reactions. The standard enthalpy of formation of any element in its most stable form is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Standard Enthalpy Of Formation Is Zero For Zno.
From www.chegg.com
Solved Calculate the Standard Enthalpy of Formation of NOCl Standard Enthalpy Of Formation Is Zero For Zno Why is that a convention? Characteristics of enthalpy of formation. The enthalpy difference between graphite and diamond is too large for both to have a standard enthalpy of formation of zero. For an element the standard state is the form in which the element exists (is more stable) under condition of 1 bar and at the temperature of. Most values. Standard Enthalpy Of Formation Is Zero For Zno.
From www.numerade.com
SOLVED The standard enthalpy of combustion of ethene gas, C₂H4 (g), is Standard Enthalpy Of Formation Is Zero For Zno Characteristics of enthalpy of formation. Most values of standard enthalpy of formation are negative, indicating that these compounds are formed through exothermic reactions. 193 rows the change in enthalpy for a reaction can be calculated from the enthalpies of formation of the reactants and the products. For an element the standard state is the form in which the element exists. Standard Enthalpy Of Formation Is Zero For Zno.
From www.numerade.com
SOLVED Calculate the standard enthalpy change for the reaction at 25 ∘ Standard Enthalpy Of Formation Is Zero For Zno Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For an element the standard state is the form in which the element exists (is more stable) under condition of 1 bar and at the temperature of. The standard enthalpy of formation of any element in its most stable. Standard Enthalpy Of Formation Is Zero For Zno.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Is Zero For Zno Most values of standard enthalpy of formation are negative, indicating that these compounds are formed through exothermic reactions. The standard enthalpy of formation of any element in its most stable form is zero by definition. The enthalpy difference between graphite and diamond is too large for both to have a standard enthalpy of formation of zero. Why is that a. Standard Enthalpy Of Formation Is Zero For Zno.
From www.numerade.com
SOLVED Exercise EZC.2(a) The standard enthalpy of formation of Standard Enthalpy Of Formation Is Zero For Zno The standard enthalpy of formation of any element in its most stable form is zero by definition. Characteristics of enthalpy of formation. The enthalpy difference between graphite and diamond is too large for both to have a standard enthalpy of formation of zero. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Standard Enthalpy Of Formation Is Zero For Zno.
From kunduz.com
[ANSWERED] 61 180 Standard molar enthalpy of formation at 298 K is zero Standard Enthalpy Of Formation Is Zero For Zno For an element the standard state is the form in which the element exists (is more stable) under condition of 1 bar and at the temperature of. Most values of standard enthalpy of formation are negative, indicating that these compounds are formed through exothermic reactions. The enthalpy difference between graphite and diamond is too large for both to have a. Standard Enthalpy Of Formation Is Zero For Zno.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of Cl(aq) given Standard Enthalpy Of Formation Is Zero For Zno For an element the standard state is the form in which the element exists (is more stable) under condition of 1 bar and at the temperature of. Characteristics of enthalpy of formation. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows the change in enthalpy for. Standard Enthalpy Of Formation Is Zero For Zno.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Is Zero For Zno Characteristics of enthalpy of formation. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows the change in enthalpy for a reaction can be calculated from the enthalpies of formation of the reactants and the products. The standard enthalpy of formation $\delta h_f^°$ of pure elements is. Standard Enthalpy Of Formation Is Zero For Zno.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Is Zero For Zno Characteristics of enthalpy of formation. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Why is that a convention? Most values of standard enthalpy of formation are negative, indicating that these compounds are formed. Standard Enthalpy Of Formation Is Zero For Zno.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Is Zero For Zno The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Why is that a convention? The standard enthalpy of formation of any element in its standard state is. 193 rows the change in enthalpy for. Standard Enthalpy Of Formation Is Zero For Zno.
From storage.googleapis.com
Standard Enthalpy Of Formation Sulfuric Acid Standard Enthalpy Of Formation Is Zero For Zno The standard enthalpy of formation of any element in its most stable form is zero by definition. The enthalpy difference between graphite and diamond is too large for both to have a standard enthalpy of formation of zero. The standard enthalpy of formation of any element in its standard state is. Why is that a convention? Standard enthalpy change of. Standard Enthalpy Of Formation Is Zero For Zno.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Is Zero For Zno The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Most values of standard enthalpy of formation are negative, indicating that these compounds are formed through exothermic reactions. Characteristics of enthalpy of formation. The enthalpy. Standard Enthalpy Of Formation Is Zero For Zno.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Is Zero For Zno Why is that a convention? Characteristics of enthalpy of formation. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows the change in enthalpy for a reaction can be calculated from the enthalpies of formation of the reactants and the products. For an element the standard state. Standard Enthalpy Of Formation Is Zero For Zno.
From kaden-chapter.blogspot.com
Standard Enthalpy Of Formation Table Pdf 30+ Pages Summary [1.8mb Standard Enthalpy Of Formation Is Zero For Zno Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For an element the standard state is the form in which the element exists (is more stable) under condition of 1 bar and at the temperature of. The standard enthalpy of formation of any element in its most stable. Standard Enthalpy Of Formation Is Zero For Zno.
From www.numerade.com
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 Standard Enthalpy Of Formation Is Zero For Zno The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. Characteristics of enthalpy of formation. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element in its standard state is. 193 rows the change in enthalpy for. Standard Enthalpy Of Formation Is Zero For Zno.
From www.toppr.com
R TYPES OF ENTHALPY OF REACTION The standard enthalpies of formation Standard Enthalpy Of Formation Is Zero For Zno For an element the standard state is the form in which the element exists (is more stable) under condition of 1 bar and at the temperature of. Characteristics of enthalpy of formation. The standard enthalpy of formation of any element in its most stable form is zero by definition. 193 rows the change in enthalpy for a reaction can be. Standard Enthalpy Of Formation Is Zero For Zno.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Is Zero For Zno Most values of standard enthalpy of formation are negative, indicating that these compounds are formed through exothermic reactions. Characteristics of enthalpy of formation. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. The standard enthalpy of formation of any element in its standard state is. Standard enthalpy change of formation (data table) these tables include. Standard Enthalpy Of Formation Is Zero For Zno.
From www.numerade.com
SOLVED 1)Baking soda (NaHCO3) on heating as follows 2 Standard Enthalpy Of Formation Is Zero For Zno 193 rows the change in enthalpy for a reaction can be calculated from the enthalpies of formation of the reactants and the products. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Most values. Standard Enthalpy Of Formation Is Zero For Zno.
From www.numerade.com
SOLVED Using 'the standard enthalpies of formation, what is the Standard Enthalpy Of Formation Is Zero For Zno The standard enthalpy of formation of any element in its most stable form is zero by definition. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. Why is that a convention? The enthalpy difference between graphite and diamond is too large for both to have a standard enthalpy of formation of zero. Characteristics of enthalpy. Standard Enthalpy Of Formation Is Zero For Zno.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Is Zero For Zno The standard enthalpy of formation of any element in its most stable form is zero by definition. For an element the standard state is the form in which the element exists (is more stable) under condition of 1 bar and at the temperature of. The standard enthalpy of formation of any element in its standard state is. The standard enthalpy. Standard Enthalpy Of Formation Is Zero For Zno.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Enthalpy Of Formation Is Zero For Zno The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. Why is that a convention? 193 rows the change in enthalpy for a reaction can be calculated from the enthalpies of formation of the reactants and the products. For an element the standard state is the form in which the element exists (is more stable) under. Standard Enthalpy Of Formation Is Zero For Zno.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Is Zero For Zno Why is that a convention? The enthalpy difference between graphite and diamond is too large for both to have a standard enthalpy of formation of zero. The standard enthalpy of formation of any element in its standard state is. 193 rows the change in enthalpy for a reaction can be calculated from the enthalpies of formation of the reactants and. Standard Enthalpy Of Formation Is Zero For Zno.
From www.numerade.com
SOLVED Consider the following table of Standard Enthalpies of Standard Enthalpy Of Formation Is Zero For Zno Why is that a convention? The enthalpy difference between graphite and diamond is too large for both to have a standard enthalpy of formation of zero. Characteristics of enthalpy of formation. Most values of standard enthalpy of formation are negative, indicating that these compounds are formed through exothermic reactions. For an element the standard state is the form in which. Standard Enthalpy Of Formation Is Zero For Zno.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Enthalpy Of Formation Is Zero For Zno Why is that a convention? The standard enthalpy of formation of any element in its standard state is. The enthalpy difference between graphite and diamond is too large for both to have a standard enthalpy of formation of zero. 193 rows the change in enthalpy for a reaction can be calculated from the enthalpies of formation of the reactants and. Standard Enthalpy Of Formation Is Zero For Zno.
From www.solutionspile.com
[Solved] Using the standard enthalpies of formation liste Standard Enthalpy Of Formation Is Zero For Zno The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. 193 rows the change in enthalpy for a reaction can be calculated from the enthalpies of formation of the reactants and the products. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Why is. Standard Enthalpy Of Formation Is Zero For Zno.