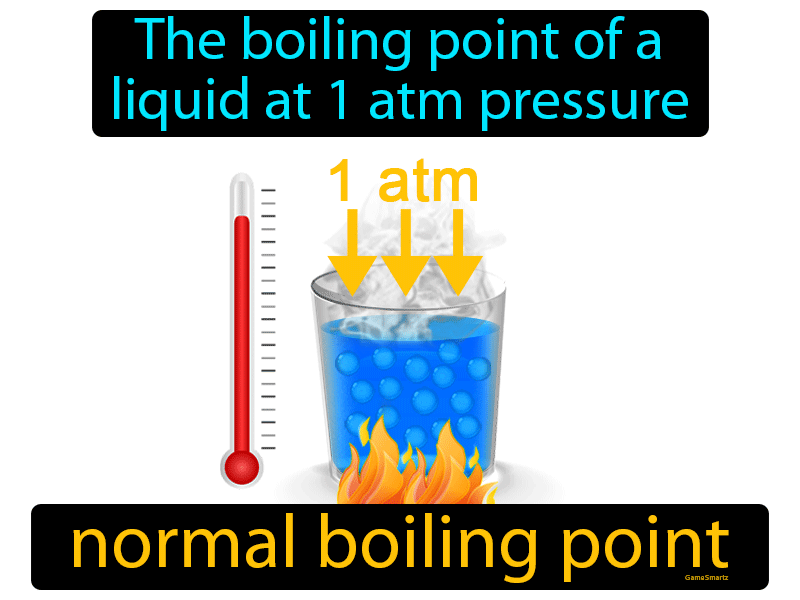
Boiling Point Of Def . The “normal” refers to sea level or an elevation of 0 meters or feet. In more technical terms, it is when a. The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. But, the boiling point of water changes with. This is the boiling point which is usually quoted in chemical literature.
from gamesmartz.com
The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The “normal” refers to sea level or an elevation of 0 meters or feet. The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. In more technical terms, it is when a. But, the boiling point of water changes with. This is the boiling point which is usually quoted in chemical literature.
Normal Boiling Point Definition & Image GameSmartz
Boiling Point Of Def The “normal” refers to sea level or an elevation of 0 meters or feet. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The “normal” refers to sea level or an elevation of 0 meters or feet. In more technical terms, it is when a. The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. This is the boiling point which is usually quoted in chemical literature. But, the boiling point of water changes with. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Boiling Point Of Def This is the boiling point which is usually quoted in chemical literature. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. In more technical terms, it is when a. The “normal” refers to sea level or an elevation of 0 meters or feet. The boiling point of a substance is the temperature at which the. Boiling Point Of Def.
From www.masterorganicchemistry.com
3 Trends That Affect Boiling Points — Master Organic Chemistry Boiling Point Of Def The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. In more technical terms, it is when a. But, the boiling point of water changes with. This is. Boiling Point Of Def.
From www.studocu.com
Boiling Point Determination PreLab Questions The boiling point of a Boiling Point Of Def The “normal” refers to sea level or an elevation of 0 meters or feet. The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. But, the boiling point of water changes with. If this pressure is the standard pressure of 1 atm (101.3. Boiling Point Of Def.
From hobbybrennen.ch
Boiling Point Diagram Generator Boiling Point Of Def But, the boiling point of water changes with. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The “normal” refers to sea level or an elevation of 0 meters or feet. In more technical terms, it is when a. This is. Boiling Point Of Def.
From brainly.com
Use the dropdown menus to rank the boiling points of the following Boiling Point Of Def This is the boiling point which is usually quoted in chemical literature. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. In more technical terms, it is. Boiling Point Of Def.
From slideplayer.com
Energy Changes & Phase Changes ppt download Boiling Point Of Def The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The “normal” refers to sea level or an elevation of 0 meters or feet. The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. But, the boiling point of. Boiling Point Of Def.
From slideplayer.com
Matter. ppt download Boiling Point Of Def But, the boiling point of water changes with. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. In more technical terms, it is when a. The “normal” refers to sea level or an elevation of 0 meters or feet. This is. Boiling Point Of Def.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Boiling Point Of Def The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. But, the boiling point of water changes with. This is the boiling point which is usually quoted in chemical literature. In more technical terms, it is when a. The “normal” refers to sea. Boiling Point Of Def.
From whistlecleanaustralia.com.au
Boiling Point Whistle Clean Australia Boiling Point Of Def The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. This is the boiling point which is usually quoted in chemical literature. But, the boiling point of water changes with. In more technical terms, it is when a. The normal boiling point of. Boiling Point Of Def.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Boiling Point Of Def But, the boiling point of water changes with. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a. Boiling Point Of Def.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point Boiling Point Of Def In more technical terms, it is when a. The “normal” refers to sea level or an elevation of 0 meters or feet. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. This is the boiling point which is usually quoted in chemical literature. The boiling point of a substance is the temperature at which the. Boiling Point Of Def.
From www.coursehero.com
[Solved] Which of these compounds will have the highest boiling point Boiling Point Of Def The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The “normal” refers to sea. Boiling Point Of Def.
From www.coursehero.com
[Solved] (i) The boiling point of dinbutyldivinyltin at 10 mmHg is Boiling Point Of Def The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. But, the boiling point of water changes with. The “normal” refers to sea level or an elevation of 0 meters or feet. The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a. Boiling Point Of Def.
From www.tessshebaylo.com
Chemical Equation For Water Boiling Point Tessshebaylo Boiling Point Of Def The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The “normal” refers to sea level or an elevation of 0 meters or feet. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. In more technical terms,. Boiling Point Of Def.
From www.yiannislucacos.gr
How to boil Boiling as a basic cooking method Yiannis Lucacos Boiling Point Of Def If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The boiling point of a substance is the temperature at which the liquid and vapour phase of the. Boiling Point Of Def.
From brainly.com
This graph shows the melting and boiling points of the alkali metals Boiling Point Of Def If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. But, the boiling point of water changes with. In more technical terms, it is when a. This is the boiling point which is usually quoted in chemical literature. The boiling point of. Boiling Point Of Def.
From slideplayer.com
Physical and Chemical Properties ppt download Boiling Point Of Def This is the boiling point which is usually quoted in chemical literature. The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. Boiling Point Of Def.
From www.masterorganicchemistry.com
3 Trends That Affect Boiling Points — Master Organic Chemistry Boiling Point Of Def If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. In more technical terms, it. Boiling Point Of Def.
From www.researchgate.net
Extraction at boiling point of solvents Download Scientific Diagram Boiling Point Of Def In more technical terms, it is when a. The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. If this pressure is the standard pressure of 1 atm (101.3. Boiling Point Of Def.
From www.liveworksheets.com
Melting and Boiling Point Alex Crentsil Live Boiling Point Of Def If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The “normal” refers to sea level or an elevation of 0 meters or feet. In more technical terms,. Boiling Point Of Def.
From elecschem.com
The Importance of Understanding Boiling Point Diagrams for Chemical Boiling Point Of Def If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. This is the boiling point which is usually quoted in chemical literature. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. In more technical terms, it is. Boiling Point Of Def.
From www.youtube.com
Calculating boiling point elevation YouTube Boiling Point Of Def If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. The normal boiling point of. Boiling Point Of Def.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Boiling Point Of Def But, the boiling point of water changes with. The “normal” refers to sea level or an elevation of 0 meters or feet. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. In more technical terms, it is when a. The normal. Boiling Point Of Def.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting Boiling Point Of Def The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. This is the boiling point which is usually quoted in chemical literature. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. Boiling Point Of Def.
From jsmithmoore.com
Boiling point of ethanol celsius Boiling Point Of Def But, the boiling point of water changes with. This is the boiling point which is usually quoted in chemical literature. The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. The “normal” refers to sea level or an elevation of 0 meters or. Boiling Point Of Def.
From gamesmartz.com
Boiling Point Definition & Image GameSmartz Boiling Point Of Def If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The boiling point of a substance is the temperature at which the liquid and vapour phase of the. Boiling Point Of Def.
From gamesmartz.com
Normal Boiling Point Definition & Image GameSmartz Boiling Point Of Def The “normal” refers to sea level or an elevation of 0 meters or feet. But, the boiling point of water changes with. In more technical terms, it is when a. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The boiling. Boiling Point Of Def.
From slideplayer.com
Water and Aqueous Systems ppt download Boiling Point Of Def The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. In more technical terms, it is when a. The “normal” refers to sea level or an elevation of. Boiling Point Of Def.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Boiling Point Of Def But, the boiling point of water changes with. The “normal” refers to sea level or an elevation of 0 meters or feet. This is the boiling point which is usually quoted in chemical literature. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The boiling point of a substance is the temperature at which the. Boiling Point Of Def.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point Boiling Point Of Def The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. This is the boiling point which is usually quoted in chemical literature. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. In more technical terms, it is when. Boiling Point Of Def.
From www.youtube.com
Boiling/Melting Points and Intermolecular Forces YouTube Boiling Point Of Def The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. But, the boiling point of water changes with. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its. Boiling Point Of Def.
From www.studocu.com
Determination of boiling point organic Determination of boiling point Boiling Point Of Def The “normal” refers to sea level or an elevation of 0 meters or feet. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The boiling point of a substance is the temperature at which the liquid and vapour phase of the. Boiling Point Of Def.
From theteastylist.com
The Boiling Point The Tea Stylist Boiling Point Of Def This is the boiling point which is usually quoted in chemical literature. But, the boiling point of water changes with. The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. If this pressure is the standard pressure of 1 atm (101.3 kpa), then. Boiling Point Of Def.
From ar.inspiredpencil.com
Boiling Point Elevation Equation Boiling Point Of Def The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The normal boiling point of. Boiling Point Of Def.
From pubchem.ncbi.nlm.nih.gov
Boiling Point Periodic Table of Elements PubChem Boiling Point Of Def If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The boiling point of a substance is the temperature at which the liquid and vapour phase of the substance exists in equilibrium for a specific pressure condition. In more technical terms, it. Boiling Point Of Def.