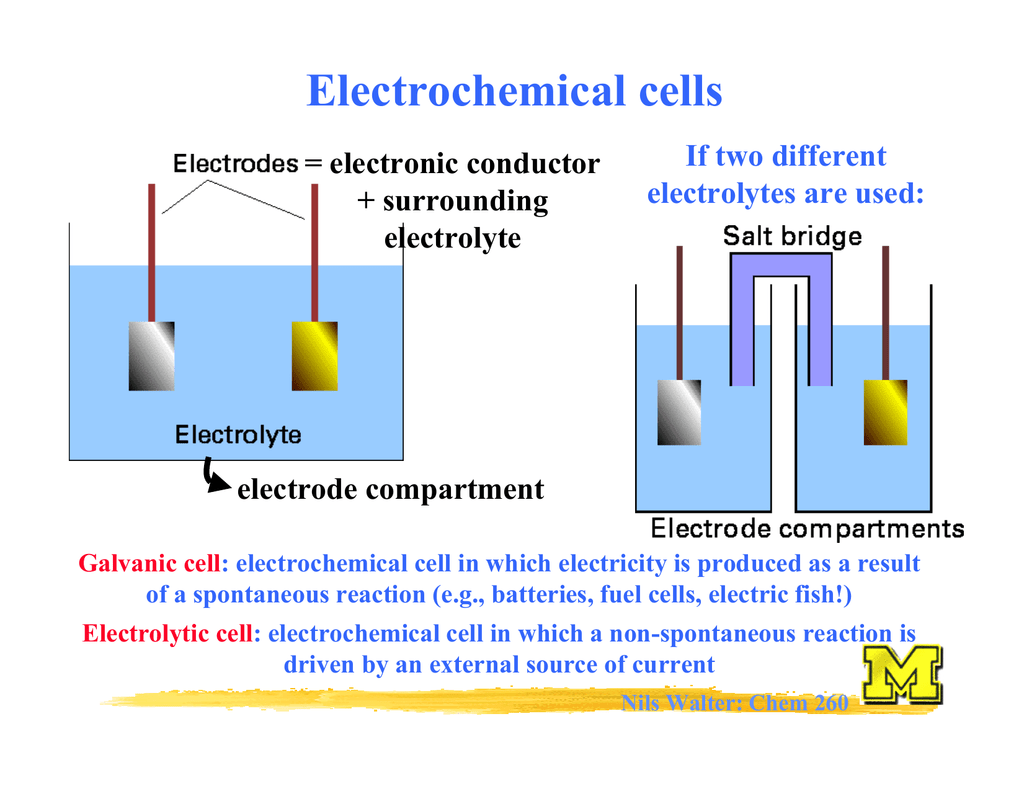
Electrochemical Reaction Example . An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very useful molecules, from water. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electricity, which is produced by the transfer of electrons from one element. Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by movements of. Electrochemistry deals with chemical reactions that produce electricity and the changes associated with the passage of. The study of chemical reactions that move electrons is known as electrochemistry. This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied.
from studylib.net
An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very useful molecules, from water. Electrochemistry deals with chemical reactions that produce electricity and the changes associated with the passage of. The study of chemical reactions that move electrons is known as electrochemistry. Electrochemistry is the study of chemical processes that cause electrons to move. Electricity, which is produced by the transfer of electrons from one element. This movement of electrons is called electricity, which can be generated by movements of.
Electrochemical cells
Electrochemical Reaction Example This movement of electrons is called electricity, which can be generated by movements of. This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. Electricity, which is produced by the transfer of electrons from one element. Electrochemistry deals with chemical reactions that produce electricity and the changes associated with the passage of. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. This movement of electrons is called electricity, which can be generated by movements of. Electrochemistry is the study of chemical processes that cause electrons to move. The study of chemical reactions that move electrons is known as electrochemistry. An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very useful molecules, from water.
From courses.lumenlearning.com
Electrolysis Chemistry Electrochemical Reaction Example This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. Electrochemistry is the study of chemical processes that cause electrons to move. Electricity, which is produced by the transfer of electrons from one element. The study of chemical reactions that move electrons is known as electrochemistry. An example is the reaction 2h 2 o →. Electrochemical Reaction Example.
From wisc.pb.unizin.org
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109 Electrochemical Reaction Example Electricity, which is produced by the transfer of electrons from one element. An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very useful molecules, from water. This movement of electrons is called electricity, which can be generated by movements of. The study of chemical reactions that move electrons. Electrochemical Reaction Example.
From pubs.acs.org
Electrochemical Arylation Reaction Chemical Reviews Electrochemical Reaction Example Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemistry deals with chemical reactions that produce electricity and the changes associated with the passage of. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. The study of chemical reactions that move electrons is known as electrochemistry. This decomposition does. Electrochemical Reaction Example.
From studylib.net
Electrochemical cells Electrochemical Reaction Example This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by movements of. The study. Electrochemical Reaction Example.
From www.thoughtco.com
Electrochemical Cell Definition Electrochemical Reaction Example This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemistry deals with chemical reactions that produce electricity and the changes associated with the passage of. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into.. Electrochemical Reaction Example.
From generic.wordpress.soton.ac.uk
Electrochemistry explanations, videos and everyday life examples Electrochemical Reaction Example Electrochemistry deals with chemical reactions that produce electricity and the changes associated with the passage of. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. The study of chemical reactions that move electrons is known as electrochemistry. Electricity, which is produced by the transfer of electrons from one element. This decomposition does. Electrochemical Reaction Example.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps Electrochemical Reaction Example The study of chemical reactions that move electrons is known as electrochemistry. An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very useful molecules, from water. This movement of electrons is called electricity, which can be generated by movements of. An electrochemical cell is any device that converts. Electrochemical Reaction Example.
From www.youtube.com
Emf of daniel cell from nernst equation(Electrochemistry part 28 for Electrochemical Reaction Example Electrochemistry deals with chemical reactions that produce electricity and the changes associated with the passage of. Electricity, which is produced by the transfer of electrons from one element. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. This movement of electrons is called electricity, which can be generated by movements of. This. Electrochemical Reaction Example.
From pubs.acs.org
Electrochemical Strategy for the Simultaneous Production of Electrochemical Reaction Example An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very useful molecules, from water. This movement of electrons is called electricity, which can be generated by movements of. The study of. Electrochemical Reaction Example.
From www.researchgate.net
Example of a SOC with the corresponding electrochemical reactions for Electrochemical Reaction Example An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemistry is the study of chemical processes that cause electrons to move. The study of chemical reactions that move electrons is known as electrochemistry. This movement of electrons is called electricity, which can be generated by movements of. This decomposition does not occur. Electrochemical Reaction Example.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Electrochemical Reaction Example Electrochemistry is the study of chemical processes that cause electrons to move. Electricity, which is produced by the transfer of electrons from one element. This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two. Electrochemical Reaction Example.
From saylordotorg.github.io
Electrochemistry Electrochemical Reaction Example An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electricity, which is produced by the transfer of electrons from one element. Electrochemistry is the study of chemical processes that cause electrons to move. An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen. Electrochemical Reaction Example.
From www.pinterest.com
Electrochemical cell Electrochemistry, Chemistry classroom Electrochemical Reaction Example This movement of electrons is called electricity, which can be generated by movements of. Electricity, which is produced by the transfer of electrons from one element. The study of chemical reactions that move electrons is known as electrochemistry. Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemistry deals with chemical reactions that produce electricity and the. Electrochemical Reaction Example.
From www.dynamicscience.com.au
redox reactions electrolysis The difference between electrochemical Electrochemical Reaction Example This movement of electrons is called electricity, which can be generated by movements of. This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. The study of chemical reactions that move electrons is known as electrochemistry. Electricity, which is produced by the transfer of electrons from one element. Electrochemistry deals with chemical reactions that produce. Electrochemical Reaction Example.
From brainly.in
example of photochemical reaction and electrochemical reaction Brainly.in Electrochemical Reaction Example This movement of electrons is called electricity, which can be generated by movements of. This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. Electricity, which is produced by the transfer of electrons from one element. Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemistry deals with chemical reactions that produce. Electrochemical Reaction Example.
From www.researchgate.net
Scheme 1 Schematic representation of the electrochemical reaction of Electrochemical Reaction Example An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very useful molecules, from water. Electricity, which is produced by the transfer of electrons from one element. This movement of electrons is called electricity, which can be generated by movements of. An electrochemical cell is any device that converts. Electrochemical Reaction Example.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson Electrochemical Reaction Example This movement of electrons is called electricity, which can be generated by movements of. This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. The study of chemical reactions that move electrons is known as electrochemistry. Electrochemistry is the study of chemical processes that cause electrons to move. Electricity, which is produced by the transfer. Electrochemical Reaction Example.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Electrochemical Reaction Example An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemistry deals with chemical reactions that produce electricity and the changes associated with the passage of. This movement of electrons is called electricity, which can be generated by movements of. Electrochemistry is the study of chemical processes that cause electrons to move. The. Electrochemical Reaction Example.
From www.researchgate.net
Schematic of the electrochemical reaction occurring inside the VIA Electrochemical Reaction Example Electrochemistry deals with chemical reactions that produce electricity and the changes associated with the passage of. The study of chemical reactions that move electrons is known as electrochemistry. This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. Electrochemistry is the study of chemical processes that cause electrons to move. Electricity, which is produced by. Electrochemical Reaction Example.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID541675 Electrochemical Reaction Example This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemistry deals with chemical reactions that produce electricity and the changes associated with the passage of. This movement of electrons is called electricity, which can be generated by. Electrochemical Reaction Example.
From slidetodoc.com
Chapter 20 Electrochemistry Electrochemical Reactions In Electrochemical Reaction Example This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. Electrochemistry is the study of chemical processes that cause electrons to move. The study of chemical reactions that move electrons is known as electrochemistry. Electrochemistry deals with chemical reactions that produce electricity and the changes associated with the passage of. An example is the reaction. Electrochemical Reaction Example.
From peoi.org
Chapter 14 Section C Applications of Redox Reactions Voltaic Cells Electrochemical Reaction Example This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very useful molecules, from water. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electricity, which. Electrochemical Reaction Example.
From 2012books.lardbucket.org
Electrochemistry Electrochemical Reaction Example Electrochemistry deals with chemical reactions that produce electricity and the changes associated with the passage of. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. This movement of electrons is called electricity, which can be generated by movements of. This decomposition does not occur naturally, but readily proceeds with a relatively small. Electrochemical Reaction Example.
From www.slideshare.net
Electrochemicalreactions Electrochemical Reaction Example Electrochemistry is the study of chemical processes that cause electrons to move. An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very useful molecules, from water. Electricity, which is produced by the transfer of electrons from one element. An electrochemical cell is any device that converts chemical energy. Electrochemical Reaction Example.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrochemical Reaction Example An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very useful molecules, from water. The study of chemical reactions that move electrons is known as electrochemistry. Electricity, which is produced by the transfer of electrons from one element. This decomposition does not occur naturally, but readily proceeds with. Electrochemical Reaction Example.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrochemical Reaction Example Electrochemistry is the study of chemical processes that cause electrons to move. Electricity, which is produced by the transfer of electrons from one element. This movement of electrons is called electricity, which can be generated by movements of. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. This decomposition does not occur. Electrochemical Reaction Example.
From marco-has-branch.blogspot.com
Which Statement Describes the Reactions in an Electrochemical Cell Electrochemical Reaction Example An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. This movement of electrons is called electricity, which can be generated by movements of. This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. Electricity, which is produced by the transfer of electrons from one element. Electrochemistry deals. Electrochemical Reaction Example.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and Electrochemical Reaction Example An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very useful molecules, from water. This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. The study of chemical reactions that move electrons is known as electrochemistry. An electrochemical cell is any device that. Electrochemical Reaction Example.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrochemical Reaction Example Electrochemistry is the study of chemical processes that cause electrons to move. Electricity, which is produced by the transfer of electrons from one element. This movement of electrons is called electricity, which can be generated by movements of. An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very. Electrochemical Reaction Example.
From blog.syrris.com
Electrochemistry made easy with continuous flow chemistry techniques Electrochemical Reaction Example The study of chemical reactions that move electrons is known as electrochemistry. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. This movement of electrons is called electricity, which can be generated by movements of. Electricity, which is produced by the transfer of electrons from one element. This decomposition does not occur. Electrochemical Reaction Example.
From www.slideserve.com
PPT Introduction to electrochemical systems PowerPoint Presentation Electrochemical Reaction Example Electrochemistry deals with chemical reactions that produce electricity and the changes associated with the passage of. An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very useful molecules, from water. Electricity, which is produced by the transfer of electrons from one element. The study of chemical reactions that. Electrochemical Reaction Example.
From www.researchgate.net
Example of designs for the electrochemical reaction system for large Electrochemical Reaction Example This movement of electrons is called electricity, which can be generated by movements of. This decomposition does not occur naturally, but readily proceeds with a relatively small voltage applied. An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very useful molecules, from water. The study of chemical reactions. Electrochemical Reaction Example.
From mehndidesign.zohal.cc
Electrochemical Reaction Definition Process Types Examples ZOHAL Electrochemical Reaction Example The study of chemical reactions that move electrons is known as electrochemistry. This movement of electrons is called electricity, which can be generated by movements of. An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very useful molecules, from water. Electrochemistry is the study of chemical processes that. Electrochemical Reaction Example.
From pubs.rsc.org
Making electrochemistry easily accessible to the synthetic chemist Electrochemical Reaction Example An example is the reaction 2h 2 o → 2h 2 + o 2 which generates hydrogen and oxygen gas, two very useful molecules, from water. The study of chemical reactions that move electrons is known as electrochemistry. Electricity, which is produced by the transfer of electrons from one element. Electrochemistry is the study of chemical processes that cause electrons. Electrochemical Reaction Example.
From printorders.aip.org
Multiscale Modeling of Electrochemical Reactions and Processes AIP Electrochemical Reaction Example The study of chemical reactions that move electrons is known as electrochemistry. This movement of electrons is called electricity, which can be generated by movements of. Electrochemistry deals with chemical reactions that produce electricity and the changes associated with the passage of. Electricity, which is produced by the transfer of electrons from one element. An electrochemical cell is any device. Electrochemical Reaction Example.