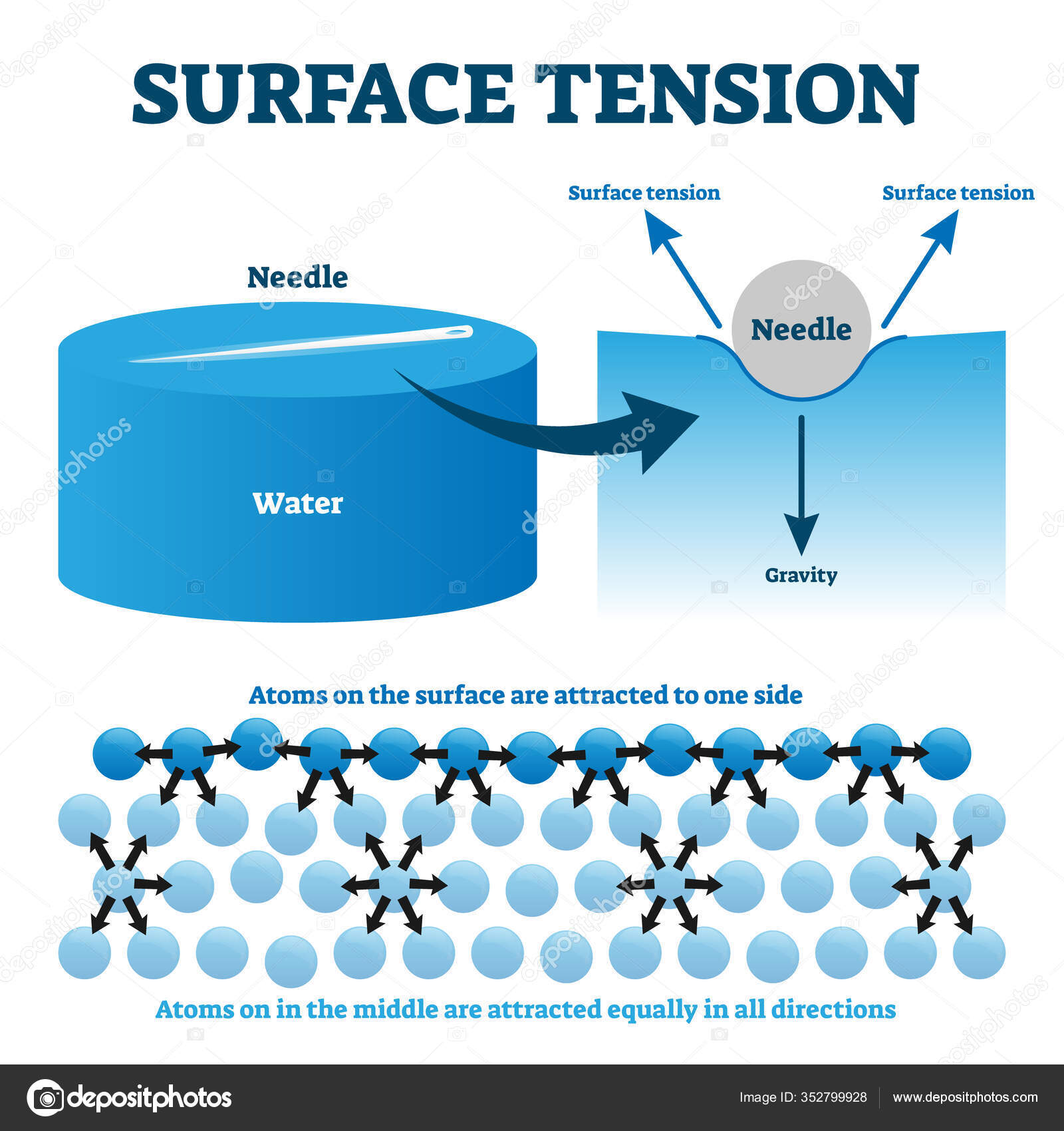
What Has More Surface Tension Water Or Isopropanol . surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is caused by the inward attraction of molecules at a boundary. • the polarity of water molecules. surface tension of liquids like water, mercury, oils and more. isopropyl alcohol has a significantly lower surface tension compared to water, which has a surface tension of 71.99 dynes/cm at 25°c. surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Water, soapy water, or rubbing alcohol. • the attraction of molecules at the surface of a liquid is called surface tension. surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and. in this portion of the lab, you will determine which liquid has the highest surface tension: To do this, you will count the.
from depositphotos.com
Surface tension is caused by the inward attraction of molecules at a boundary. To do this, you will count the. • the attraction of molecules at the surface of a liquid is called surface tension. surface tension of liquids like water, mercury, oils and more. in this portion of the lab, you will determine which liquid has the highest surface tension: • the polarity of water molecules. Water, soapy water, or rubbing alcohol. surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. isopropyl alcohol has a significantly lower surface tension compared to water, which has a surface tension of 71.99 dynes/cm at 25°c.
Surface tension explanation vector illustration diagram Stock Vector
What Has More Surface Tension Water Or Isopropanol surface tension of liquids like water, mercury, oils and more. surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. To do this, you will count the. • the attraction of molecules at the surface of a liquid is called surface tension. isopropyl alcohol has a significantly lower surface tension compared to water, which has a surface tension of 71.99 dynes/cm at 25°c. surface tension of liquids like water, mercury, oils and more. • the polarity of water molecules. surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and. in this portion of the lab, you will determine which liquid has the highest surface tension: Surface tension is caused by the inward attraction of molecules at a boundary. Water, soapy water, or rubbing alcohol.
From www.youtube.com
Surface Tension of Water Explained YouTube What Has More Surface Tension Water Or Isopropanol surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. • the polarity of water molecules. surface tension of liquids like water, mercury, oils and more. isopropyl alcohol has a significantly lower surface tension compared to water, which has a surface tension of. What Has More Surface Tension Water Or Isopropanol.
From www.vrogue.co
What Is Surface Tension Molecular Explanation vrogue.co What Has More Surface Tension Water Or Isopropanol isopropyl alcohol has a significantly lower surface tension compared to water, which has a surface tension of 71.99 dynes/cm at 25°c. • the attraction of molecules at the surface of a liquid is called surface tension. To do this, you will count the. surface tension is caused by a strong attraction between the molecules (cohesion) that cause them. What Has More Surface Tension Water Or Isopropanol.
From www.slideserve.com
PPT FLUID PROPERTIES Chapter 2 PowerPoint Presentation, free download What Has More Surface Tension Water Or Isopropanol surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. surface tension of liquids like water, mercury, oils and more. surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and. • the polarity of. What Has More Surface Tension Water Or Isopropanol.
From studyschoollifebelts.z21.web.core.windows.net
How To Explain Surface Tension What Has More Surface Tension Water Or Isopropanol Surface tension is caused by the inward attraction of molecules at a boundary. in this portion of the lab, you will determine which liquid has the highest surface tension: To do this, you will count the. isopropyl alcohol has a significantly lower surface tension compared to water, which has a surface tension of 71.99 dynes/cm at 25°c. . What Has More Surface Tension Water Or Isopropanol.
From www.slideserve.com
PPT Phases of Matter PowerPoint Presentation, free download ID898359 What Has More Surface Tension Water Or Isopropanol Surface tension is caused by the inward attraction of molecules at a boundary. surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and. Water, soapy water, or rubbing alcohol. • the attraction of molecules at the surface of a liquid is called surface tension. surface tension is the energy,. What Has More Surface Tension Water Or Isopropanol.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces What Has More Surface Tension Water Or Isopropanol Water, soapy water, or rubbing alcohol. • the polarity of water molecules. • the attraction of molecules at the surface of a liquid is called surface tension. surface tension of liquids like water, mercury, oils and more. isopropyl alcohol has a significantly lower surface tension compared to water, which has a surface tension of 71.99 dynes/cm at 25°c.. What Has More Surface Tension Water Or Isopropanol.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free What Has More Surface Tension Water Or Isopropanol surface tension of liquids like water, mercury, oils and more. Water, soapy water, or rubbing alcohol. in this portion of the lab, you will determine which liquid has the highest surface tension: surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and. • the attraction of molecules at. What Has More Surface Tension Water Or Isopropanol.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Has More Surface Tension Water Or Isopropanol in this portion of the lab, you will determine which liquid has the highest surface tension: To do this, you will count the. • the polarity of water molecules. surface tension of liquids like water, mercury, oils and more. surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together. What Has More Surface Tension Water Or Isopropanol.
From ar.inspiredpencil.com
Surface Tension Of Water Molecules What Has More Surface Tension Water Or Isopropanol surface tension of liquids like water, mercury, oils and more. Water, soapy water, or rubbing alcohol. isopropyl alcohol has a significantly lower surface tension compared to water, which has a surface tension of 71.99 dynes/cm at 25°c. To do this, you will count the. in this portion of the lab, you will determine which liquid has the. What Has More Surface Tension Water Or Isopropanol.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii What Has More Surface Tension Water Or Isopropanol Water, soapy water, or rubbing alcohol. surface tension of liquids like water, mercury, oils and more. Surface tension is caused by the inward attraction of molecules at a boundary. surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. • the attraction of molecules. What Has More Surface Tension Water Or Isopropanol.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation ID565121 What Has More Surface Tension Water Or Isopropanol Water, soapy water, or rubbing alcohol. in this portion of the lab, you will determine which liquid has the highest surface tension: surface tension of liquids like water, mercury, oils and more. surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and. surface tension is the energy,. What Has More Surface Tension Water Or Isopropanol.
From circuitwiringtray.z13.web.core.windows.net
Surface Tension Of Water Diagram What Has More Surface Tension Water Or Isopropanol isopropyl alcohol has a significantly lower surface tension compared to water, which has a surface tension of 71.99 dynes/cm at 25°c. Water, soapy water, or rubbing alcohol. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is the energy required to increase the surface. What Has More Surface Tension Water Or Isopropanol.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) What Has More Surface Tension Water Or Isopropanol surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. in this portion of the lab, you will determine which liquid has the highest surface tension: Surface tension is caused by the inward attraction of molecules at a boundary. Water, soapy water, or rubbing. What Has More Surface Tension Water Or Isopropanol.
From dxozbnpql.blob.core.windows.net
Surface Tension In Water Definition at William Griffin blog What Has More Surface Tension Water Or Isopropanol To do this, you will count the. • the attraction of molecules at the surface of a liquid is called surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to. What Has More Surface Tension Water Or Isopropanol.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What Has More Surface Tension Water Or Isopropanol Water, soapy water, or rubbing alcohol. To do this, you will count the. surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. • the attraction of molecules at the surface of a liquid is called surface tension. • the polarity of water molecules. . What Has More Surface Tension Water Or Isopropanol.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What Has More Surface Tension Water Or Isopropanol surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. surface tension of liquids like water, mercury, oils and more. in this portion of the lab, you will determine which liquid has the highest surface tension: To do this, you will count the.. What Has More Surface Tension Water Or Isopropanol.
From www.biolinscientific.com
Surface tension of water Why is it so high? What Has More Surface Tension Water Or Isopropanol surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and. in this portion of the lab, you will determine which liquid has the highest surface tension: • the attraction of molecules at the surface of a liquid is called surface tension. surface tension is the energy required to. What Has More Surface Tension Water Or Isopropanol.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Has More Surface Tension Water Or Isopropanol surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Surface tension is caused by the inward attraction of molecules at a boundary. in this portion of the lab, you will determine which liquid has the highest surface tension: surface tension is caused. What Has More Surface Tension Water Or Isopropanol.
From www.researchgate.net
Surface tension dependences of isopropyl alcohol (IPA), ethanol What Has More Surface Tension Water Or Isopropanol • the polarity of water molecules. Surface tension is caused by the inward attraction of molecules at a boundary. To do this, you will count the. in this portion of the lab, you will determine which liquid has the highest surface tension: surface tension is the energy, or work, required to increase the surface area of a liquid. What Has More Surface Tension Water Or Isopropanol.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Has More Surface Tension Water Or Isopropanol • the polarity of water molecules. To do this, you will count the. surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Surface tension is caused by the inward attraction of molecules at a boundary. surface tension is caused by a strong attraction. What Has More Surface Tension Water Or Isopropanol.
From www.researchgate.net
2 Schematic representation of surface tension of a hemispherical water What Has More Surface Tension Water Or Isopropanol surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. isopropyl alcohol has a significantly lower surface tension compared to water, which has a surface tension of 71.99 dynes/cm at 25°c. To do this, you will count the. • the attraction of molecules at. What Has More Surface Tension Water Or Isopropanol.
From exolnazbp.blob.core.windows.net
Water Surface Tension With Temperature at Amber Holmes blog What Has More Surface Tension Water Or Isopropanol surface tension of liquids like water, mercury, oils and more. Surface tension is caused by the inward attraction of molecules at a boundary. • the attraction of molecules at the surface of a liquid is called surface tension. • the polarity of water molecules. isopropyl alcohol has a significantly lower surface tension compared to water, which has a. What Has More Surface Tension Water Or Isopropanol.
From eduinput.com
Surface TensionDefinition, Examples, Causes, And Measurement What Has More Surface Tension Water Or Isopropanol To do this, you will count the. surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. surface tension of liquids like water, mercury, oils and more. Water, soapy water, or rubbing alcohol. surface tension is caused by a strong attraction between the. What Has More Surface Tension Water Or Isopropanol.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint What Has More Surface Tension Water Or Isopropanol surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and. isopropyl alcohol has a significantly lower surface tension compared to water, which has a surface tension of 71.99 dynes/cm at 25°c. Surface tension is caused by the inward attraction of molecules at a boundary. • the attraction of molecules. What Has More Surface Tension Water Or Isopropanol.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What Has More Surface Tension Water Or Isopropanol surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and. • the polarity of water molecules. surface tension of liquids like water, mercury, oils and more. • the attraction of molecules at the surface of a liquid is called surface tension. in this portion of the lab, you. What Has More Surface Tension Water Or Isopropanol.
From slideplayer.com
Chemistry Review and Properties of Water! ppt download What Has More Surface Tension Water Or Isopropanol • the attraction of molecules at the surface of a liquid is called surface tension. surface tension of liquids like water, mercury, oils and more. Water, soapy water, or rubbing alcohol. Surface tension is caused by the inward attraction of molecules at a boundary. surface tension is the energy, or work, required to increase the surface area of. What Has More Surface Tension Water Or Isopropanol.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse What Has More Surface Tension Water Or Isopropanol • the attraction of molecules at the surface of a liquid is called surface tension. surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. surface tension of liquids like water, mercury, oils and more. To do this, you will count the. isopropyl. What Has More Surface Tension Water Or Isopropanol.
From www.slideserve.com
PPT Unit 4 The Hydrosphere PowerPoint Presentation, free download What Has More Surface Tension Water Or Isopropanol surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and. • the polarity of water molecules. isopropyl alcohol has a significantly lower surface tension compared to water, which has a surface tension of 71.99 dynes/cm at 25°c. surface tension of liquids like water, mercury, oils and more. To. What Has More Surface Tension Water Or Isopropanol.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson What Has More Surface Tension Water Or Isopropanol surface tension of liquids like water, mercury, oils and more. in this portion of the lab, you will determine which liquid has the highest surface tension: Surface tension is caused by the inward attraction of molecules at a boundary. • the polarity of water molecules. Water, soapy water, or rubbing alcohol. • the attraction of molecules at the. What Has More Surface Tension Water Or Isopropanol.
From www.slideserve.com
PPT Chapter 17 “Water and Aqueous Systems” PowerPoint Presentation What Has More Surface Tension Water Or Isopropanol in this portion of the lab, you will determine which liquid has the highest surface tension: surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water, soapy water, or rubbing alcohol. surface tension of liquids like water, mercury, oils and more. Surface tension is caused by. What Has More Surface Tension Water Or Isopropanol.
From slideplayer.com
Chapter 15 “Water and Aqueous Systems” ppt download What Has More Surface Tension Water Or Isopropanol surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. surface tension of liquids like water, mercury, oils and more. • the polarity of water molecules. • the attraction of molecules at the surface of a liquid is called surface tension. Surface tension is. What Has More Surface Tension Water Or Isopropanol.
From www.researchgate.net
Surface tension coefficients in aqueous solutions of isopropanol What Has More Surface Tension Water Or Isopropanol in this portion of the lab, you will determine which liquid has the highest surface tension: surface tension of liquids like water, mercury, oils and more. To do this, you will count the. • the attraction of molecules at the surface of a liquid is called surface tension. surface tension is the energy, or work, required to. What Has More Surface Tension Water Or Isopropanol.
From funsizephysics.com
What is Surface Tension? FunsizePhysics What Has More Surface Tension Water Or Isopropanol To do this, you will count the. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water, soapy water, or rubbing alcohol. surface tension of liquids like water, mercury, oils and more. in this portion of the lab, you will determine which liquid has the highest. What Has More Surface Tension Water Or Isopropanol.
From depositphotos.com
Surface tension explanation vector illustration diagram Stock Vector What Has More Surface Tension Water Or Isopropanol To do this, you will count the. Water, soapy water, or rubbing alcohol. • the attraction of molecules at the surface of a liquid is called surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is caused by the inward attraction of molecules at. What Has More Surface Tension Water Or Isopropanol.
From chem.libretexts.org
12.2 Some Properties of Liquids Chemistry LibreTexts What Has More Surface Tension Water Or Isopropanol in this portion of the lab, you will determine which liquid has the highest surface tension: Surface tension is caused by the inward attraction of molecules at a boundary. To do this, you will count the. • the attraction of molecules at the surface of a liquid is called surface tension. • the polarity of water molecules. surface. What Has More Surface Tension Water Or Isopropanol.