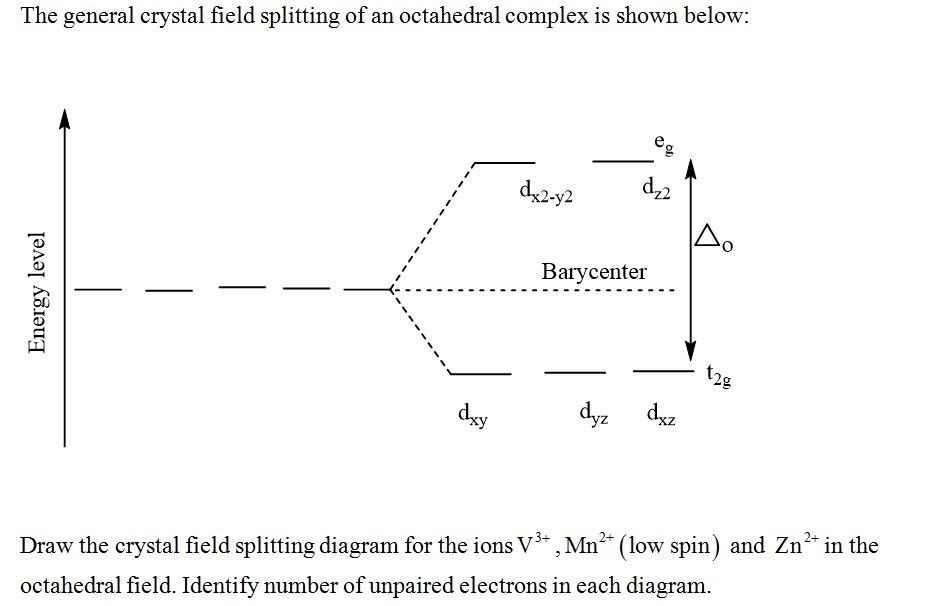
Draw Crystal Field Splitting Diagram . Draw orbital splitting diagrams for octahedral, tetrahedral and square planar geometries. Draw and label the crystal field splitting diagram ( indicating filling of electrons). Determine whether the splitting energy is greater than the pairing energy; Draw in the barycentre and label the orbitals, the. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where the subscript o stands for octahedral. Crystal field theory and its applications to metal complexes 1. Because the overall energy is maintained, the energy of the three t2g orbitals are lowered by (2/5) ∆o and the. Octahedral crystal field splitting diagram. This chemistry video tutorial provides a basic introduction into crystal field theory. It explains how to draw the crystal field splitting. The crystal field splitting in the. Draw the crystal field diagram of the complex with regards to its geometry;
from www.chegg.com
It explains how to draw the crystal field splitting. Draw and label the crystal field splitting diagram ( indicating filling of electrons). Determine whether the splitting energy is greater than the pairing energy; This chemistry video tutorial provides a basic introduction into crystal field theory. Because the overall energy is maintained, the energy of the three t2g orbitals are lowered by (2/5) ∆o and the. Draw the crystal field diagram of the complex with regards to its geometry; Octahedral crystal field splitting diagram. Draw orbital splitting diagrams for octahedral, tetrahedral and square planar geometries. Draw in the barycentre and label the orbitals, the. The crystal field splitting in the.
Solved The general crystal field splitting of an octahedral
Draw Crystal Field Splitting Diagram Draw the crystal field diagram of the complex with regards to its geometry; Draw orbital splitting diagrams for octahedral, tetrahedral and square planar geometries. Octahedral crystal field splitting diagram. Draw the crystal field diagram of the complex with regards to its geometry; Draw in the barycentre and label the orbitals, the. Because the overall energy is maintained, the energy of the three t2g orbitals are lowered by (2/5) ∆o and the. Determine whether the splitting energy is greater than the pairing energy; It explains how to draw the crystal field splitting. Draw and label the crystal field splitting diagram ( indicating filling of electrons). The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where the subscript o stands for octahedral. The crystal field splitting in the. This chemistry video tutorial provides a basic introduction into crystal field theory. Crystal field theory and its applications to metal complexes 1.
From ar.inspiredpencil.com
Trigonal Bipyramidal Crystal Field Splitting Draw Crystal Field Splitting Diagram The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where the subscript o stands for octahedral. Draw the crystal field diagram of the complex with regards to its geometry; Draw in the barycentre and label the orbitals, the. It explains how to draw the crystal field splitting. Because the. Draw Crystal Field Splitting Diagram.
From epr.ethz.ch
CrystalField Splitting and SpinOrbit Coupling Electron Draw Crystal Field Splitting Diagram The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where the subscript o stands for octahedral. Draw in the barycentre and label the orbitals, the. Draw the crystal field diagram of the complex with regards to its geometry; The crystal field splitting in the. This chemistry video tutorial provides. Draw Crystal Field Splitting Diagram.
From www.coursehero.com
[Solved] Draw a fully labelled crystal field splitting diagram for the Draw Crystal Field Splitting Diagram The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where the subscript o stands for octahedral. Because the overall energy is maintained, the energy of the three t2g orbitals are lowered by (2/5) ∆o and the. It explains how to draw the crystal field splitting. The crystal field splitting. Draw Crystal Field Splitting Diagram.
From www.researchgate.net
Schematic representation of crystal field splitting for octahedral Draw Crystal Field Splitting Diagram Crystal field theory and its applications to metal complexes 1. The crystal field splitting in the. Draw orbital splitting diagrams for octahedral, tetrahedral and square planar geometries. Determine whether the splitting energy is greater than the pairing energy; Draw the crystal field diagram of the complex with regards to its geometry; This chemistry video tutorial provides a basic introduction into. Draw Crystal Field Splitting Diagram.
From www.slideserve.com
PPT Crystal Field Theory (CFT) PowerPoint Presentation, free download Draw Crystal Field Splitting Diagram The crystal field splitting in the. Because the overall energy is maintained, the energy of the three t2g orbitals are lowered by (2/5) ∆o and the. It explains how to draw the crystal field splitting. Draw in the barycentre and label the orbitals, the. Draw orbital splitting diagrams for octahedral, tetrahedral and square planar geometries. This chemistry video tutorial provides. Draw Crystal Field Splitting Diagram.
From www.teachoo.com
[Chemistry Class 12 SQP] Draw the crystal field splitting diagram for Draw Crystal Field Splitting Diagram Draw orbital splitting diagrams for octahedral, tetrahedral and square planar geometries. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where the subscript o stands for octahedral. Because the overall energy is maintained, the energy of the three t2g orbitals are lowered by (2/5) ∆o and the. The crystal. Draw Crystal Field Splitting Diagram.
From galvinconanstuart.blogspot.com
Draw The Octahedral Crystal Field Splitting Diagram For Each Metal Ion Draw Crystal Field Splitting Diagram This chemistry video tutorial provides a basic introduction into crystal field theory. Because the overall energy is maintained, the energy of the three t2g orbitals are lowered by (2/5) ∆o and the. Draw in the barycentre and label the orbitals, the. The crystal field splitting in the. Crystal field theory and its applications to metal complexes 1. Octahedral crystal field. Draw Crystal Field Splitting Diagram.
From www.chegg.com
Solved The general crystal field splitting of an octahedral Draw Crystal Field Splitting Diagram It explains how to draw the crystal field splitting. Draw and label the crystal field splitting diagram ( indicating filling of electrons). This chemistry video tutorial provides a basic introduction into crystal field theory. Crystal field theory and its applications to metal complexes 1. The crystal field splitting in the. Draw the crystal field diagram of the complex with regards. Draw Crystal Field Splitting Diagram.
From www.researchgate.net
Illustration of crystal field splitting in TMOs. A demonstrates Draw Crystal Field Splitting Diagram This chemistry video tutorial provides a basic introduction into crystal field theory. Crystal field theory and its applications to metal complexes 1. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where the subscript o stands for octahedral. Octahedral crystal field splitting diagram. It explains how to draw the. Draw Crystal Field Splitting Diagram.
From www.numerade.com
SOLVEDDraw a crystal field splitting diagram for a trigonal planar Draw Crystal Field Splitting Diagram This chemistry video tutorial provides a basic introduction into crystal field theory. The crystal field splitting in the. Octahedral crystal field splitting diagram. Draw the crystal field diagram of the complex with regards to its geometry; It explains how to draw the crystal field splitting. Draw and label the crystal field splitting diagram ( indicating filling of electrons). Crystal field. Draw Crystal Field Splitting Diagram.
From chempedia.info
Crystal field splitting diagrams, 669 Big Chemical Encyclopedia Draw Crystal Field Splitting Diagram The crystal field splitting in the. Draw the crystal field diagram of the complex with regards to its geometry; Draw and label the crystal field splitting diagram ( indicating filling of electrons). Octahedral crystal field splitting diagram. Draw in the barycentre and label the orbitals, the. Because the overall energy is maintained, the energy of the three t2g orbitals are. Draw Crystal Field Splitting Diagram.
From stewart-switch.com
Understanding the Crystal Field Splitting Diagram A Comprehensive Guide Draw Crystal Field Splitting Diagram It explains how to draw the crystal field splitting. Draw in the barycentre and label the orbitals, the. This chemistry video tutorial provides a basic introduction into crystal field theory. Determine whether the splitting energy is greater than the pairing energy; Draw orbital splitting diagrams for octahedral, tetrahedral and square planar geometries. Because the overall energy is maintained, the energy. Draw Crystal Field Splitting Diagram.
From app.jove.com
Crystal Field Theory Tetrahedral and Square Planar Complexes Draw Crystal Field Splitting Diagram Octahedral crystal field splitting diagram. This chemistry video tutorial provides a basic introduction into crystal field theory. Draw in the barycentre and label the orbitals, the. It explains how to draw the crystal field splitting. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where the subscript o stands. Draw Crystal Field Splitting Diagram.
From chem.libretexts.org
Crystal Field Theory Chemistry LibreTexts Draw Crystal Field Splitting Diagram Because the overall energy is maintained, the energy of the three t2g orbitals are lowered by (2/5) ∆o and the. Draw the crystal field diagram of the complex with regards to its geometry; It explains how to draw the crystal field splitting. This chemistry video tutorial provides a basic introduction into crystal field theory. Draw orbital splitting diagrams for octahedral,. Draw Crystal Field Splitting Diagram.
From resolutionsforyou.com
Understanding Crystal Field Splitting Diagram for Octahedral Complexes Draw Crystal Field Splitting Diagram This chemistry video tutorial provides a basic introduction into crystal field theory. Octahedral crystal field splitting diagram. Determine whether the splitting energy is greater than the pairing energy; It explains how to draw the crystal field splitting. Crystal field theory and its applications to metal complexes 1. Because the overall energy is maintained, the energy of the three t2g orbitals. Draw Crystal Field Splitting Diagram.
From www.jove.com
Crystal Field Theory Tetrahedral and Square Planar Complexes JoVE Draw Crystal Field Splitting Diagram It explains how to draw the crystal field splitting. Draw the crystal field diagram of the complex with regards to its geometry; The crystal field splitting in the. Draw orbital splitting diagrams for octahedral, tetrahedral and square planar geometries. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where. Draw Crystal Field Splitting Diagram.
From www.zigya.com
What do you understand by crystal field splitting? Draw figure to show Draw Crystal Field Splitting Diagram Determine whether the splitting energy is greater than the pairing energy; This chemistry video tutorial provides a basic introduction into crystal field theory. Crystal field theory and its applications to metal complexes 1. Because the overall energy is maintained, the energy of the three t2g orbitals are lowered by (2/5) ∆o and the. Octahedral crystal field splitting diagram. The crystal. Draw Crystal Field Splitting Diagram.
From www.researchgate.net
Schematic illustration of crystal field splitting (CFS) of d orbitals Draw Crystal Field Splitting Diagram The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where the subscript o stands for octahedral. It explains how to draw the crystal field splitting. Determine whether the splitting energy is greater than the pairing energy; Draw the crystal field diagram of the complex with regards to its geometry;. Draw Crystal Field Splitting Diagram.
From app.jove.com
Crystal Field Theory Octahedral Complexes Concept Chemistry JoVe Draw Crystal Field Splitting Diagram It explains how to draw the crystal field splitting. Determine whether the splitting energy is greater than the pairing energy; Octahedral crystal field splitting diagram. The crystal field splitting in the. This chemistry video tutorial provides a basic introduction into crystal field theory. Because the overall energy is maintained, the energy of the three t2g orbitals are lowered by (2/5). Draw Crystal Field Splitting Diagram.
From www.numerade.com
SOLVEDDraw the octahedral crystal field splitting diagram for each Draw Crystal Field Splitting Diagram The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where the subscript o stands for octahedral. Draw in the barycentre and label the orbitals, the. Draw and label the crystal field splitting diagram ( indicating filling of electrons). Crystal field theory and its applications to metal complexes 1. Octahedral. Draw Crystal Field Splitting Diagram.
From www.numerade.com
The crystal field splitting diagram for a square pyramidal crystal Draw Crystal Field Splitting Diagram Draw in the barycentre and label the orbitals, the. Crystal field theory and its applications to metal complexes 1. It explains how to draw the crystal field splitting. This chemistry video tutorial provides a basic introduction into crystal field theory. Determine whether the splitting energy is greater than the pairing energy; The crystal field splitting in the. Draw the crystal. Draw Crystal Field Splitting Diagram.
From www.mikrora.com
Crystal Field Splitting Diagram Draw Crystal Field Splitting Diagram Crystal field theory and its applications to metal complexes 1. Draw orbital splitting diagrams for octahedral, tetrahedral and square planar geometries. Draw and label the crystal field splitting diagram ( indicating filling of electrons). The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where the subscript o stands for. Draw Crystal Field Splitting Diagram.
From www.britannica.com
Crystalfield splitting energy Britannica Draw Crystal Field Splitting Diagram Draw and label the crystal field splitting diagram ( indicating filling of electrons). Draw in the barycentre and label the orbitals, the. Crystal field theory and its applications to metal complexes 1. It explains how to draw the crystal field splitting. Determine whether the splitting energy is greater than the pairing energy; The difference in energy between the two sets. Draw Crystal Field Splitting Diagram.
From www.numerade.com
SOLVEDDraw the octahedral crystal field splitting diagram for each Draw Crystal Field Splitting Diagram Draw the crystal field diagram of the complex with regards to its geometry; The crystal field splitting in the. Draw in the barycentre and label the orbitals, the. Octahedral crystal field splitting diagram. Crystal field theory and its applications to metal complexes 1. Because the overall energy is maintained, the energy of the three t2g orbitals are lowered by (2/5). Draw Crystal Field Splitting Diagram.
From www.numerade.com
SOLVEDDraw a crystal field splitting diagram for a trigonal Draw Crystal Field Splitting Diagram It explains how to draw the crystal field splitting. Draw in the barycentre and label the orbitals, the. Draw the crystal field diagram of the complex with regards to its geometry; Determine whether the splitting energy is greater than the pairing energy; Octahedral crystal field splitting diagram. This chemistry video tutorial provides a basic introduction into crystal field theory. Draw. Draw Crystal Field Splitting Diagram.
From www.researchgate.net
Crystal field splitting diagram for divalent iron, cobalt and nickel in Draw Crystal Field Splitting Diagram The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where the subscript o stands for octahedral. Determine whether the splitting energy is greater than the pairing energy; The crystal field splitting in the. Draw and label the crystal field splitting diagram ( indicating filling of electrons). Crystal field theory. Draw Crystal Field Splitting Diagram.
From www.numerade.com
SOLVED Draw out the crystal field splitting diagram for an octahedral Draw Crystal Field Splitting Diagram Octahedral crystal field splitting diagram. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where the subscript o stands for octahedral. Draw the crystal field diagram of the complex with regards to its geometry; It explains how to draw the crystal field splitting. Draw in the barycentre and label. Draw Crystal Field Splitting Diagram.
From www.numerade.com
SOLVED Two crystal field splitting diagrams are shown below. The Draw Crystal Field Splitting Diagram Draw and label the crystal field splitting diagram ( indicating filling of electrons). Draw orbital splitting diagrams for octahedral, tetrahedral and square planar geometries. Draw in the barycentre and label the orbitals, the. Determine whether the splitting energy is greater than the pairing energy; Draw the crystal field diagram of the complex with regards to its geometry; Because the overall. Draw Crystal Field Splitting Diagram.
From www.researchgate.net
a) Splitting of the dorbital for a Cr 3+ cation in an octahedral Draw Crystal Field Splitting Diagram Because the overall energy is maintained, the energy of the three t2g orbitals are lowered by (2/5) ∆o and the. Draw orbital splitting diagrams for octahedral, tetrahedral and square planar geometries. It explains how to draw the crystal field splitting. Crystal field theory and its applications to metal complexes 1. The difference in energy between the two sets of d. Draw Crystal Field Splitting Diagram.
From diagramlibraryclopped.z19.web.core.windows.net
Crystal Field Energy Level Diagram Draw Crystal Field Splitting Diagram The crystal field splitting in the. This chemistry video tutorial provides a basic introduction into crystal field theory. Draw the crystal field diagram of the complex with regards to its geometry; Determine whether the splitting energy is greater than the pairing energy; Draw in the barycentre and label the orbitals, the. The difference in energy between the two sets of. Draw Crystal Field Splitting Diagram.
From oneclass.com
OneClass Draw and label the Crystal Field splitting diagram for the 3d Draw Crystal Field Splitting Diagram Determine whether the splitting energy is greater than the pairing energy; Draw and label the crystal field splitting diagram ( indicating filling of electrons). Draw orbital splitting diagrams for octahedral, tetrahedral and square planar geometries. Because the overall energy is maintained, the energy of the three t2g orbitals are lowered by (2/5) ∆o and the. The difference in energy between. Draw Crystal Field Splitting Diagram.
From www.youtube.com
Crystal Field Theory PartII. Splitting in Tetrahedral, Square Planar Draw Crystal Field Splitting Diagram It explains how to draw the crystal field splitting. Determine whether the splitting energy is greater than the pairing energy; Draw in the barycentre and label the orbitals, the. This chemistry video tutorial provides a basic introduction into crystal field theory. Draw orbital splitting diagrams for octahedral, tetrahedral and square planar geometries. Draw the crystal field diagram of the complex. Draw Crystal Field Splitting Diagram.
From www.researchgate.net
3 Crystal field splitting in a cubic crystal field. On the right hand Draw Crystal Field Splitting Diagram Draw in the barycentre and label the orbitals, the. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where the subscript o stands for octahedral. Determine whether the splitting energy is greater than the pairing energy; The crystal field splitting in the. Octahedral crystal field splitting diagram. Because the. Draw Crystal Field Splitting Diagram.
From chem.libretexts.org
Introduction to Crystal Field Theory Chemistry LibreTexts Draw Crystal Field Splitting Diagram It explains how to draw the crystal field splitting. Octahedral crystal field splitting diagram. Draw in the barycentre and label the orbitals, the. This chemistry video tutorial provides a basic introduction into crystal field theory. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (δ o), where the subscript o stands. Draw Crystal Field Splitting Diagram.
From ar.inspiredpencil.com
Trigonal Bipyramidal Crystal Field Splitting Draw Crystal Field Splitting Diagram Draw orbital splitting diagrams for octahedral, tetrahedral and square planar geometries. It explains how to draw the crystal field splitting. Determine whether the splitting energy is greater than the pairing energy; Because the overall energy is maintained, the energy of the three t2g orbitals are lowered by (2/5) ∆o and the. This chemistry video tutorial provides a basic introduction into. Draw Crystal Field Splitting Diagram.