Fuel Cell Characteristics . fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell is composed of three active components: In a fuel cell, hydrogen and oxygen are combined to generate. see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel cell type. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel electrode (anode), an oxidant electrode (cathode), and an.
from www.vedantu.com
fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell is composed of three active components: A fuel electrode (anode), an oxidant electrode (cathode), and an. see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel cell type. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate.
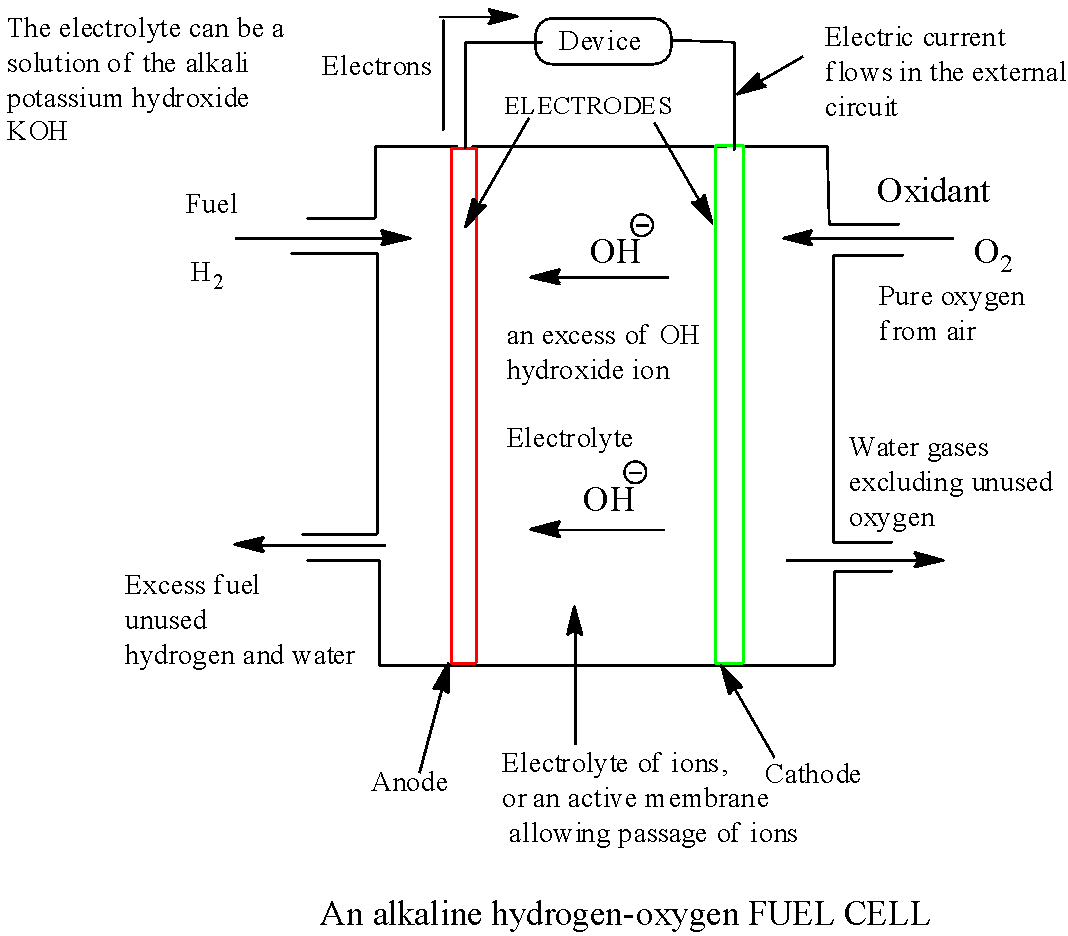
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the
Fuel Cell Characteristics A fuel electrode (anode), an oxidant electrode (cathode), and an. see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel cell type. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell is composed of three active components: fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. A fuel electrode (anode), an oxidant electrode (cathode), and an. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity.
From pubs.acs.org
Performance Characteristics of a Direct Ammonia Fuel Cell with an Anion Fuel Cell Characteristics a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity. Fuel Cell Characteristics.
From www.yourelectricalguide.com
Fuel Cell Working Principle your electrical guide Fuel Cell Characteristics see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel cell type. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently. Fuel Cell Characteristics.
From www.slideserve.com
PPT Control of Fuel Cell Power Systems PowerPoint Presentation, free Fuel Cell Characteristics A fuel electrode (anode), an oxidant electrode (cathode), and an. see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel cell type. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched. Fuel Cell Characteristics.
From www.researchgate.net
Fuel cell structure based on common LTPEMFC designs. Download Fuel Cell Characteristics A fuel electrode (anode), an oxidant electrode (cathode), and an. see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel cell type. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell is composed of. Fuel Cell Characteristics.
From www.nfcrc.uci.edu
National Fuel Cell Research Center (NFCRC), UC Irvine Fuel Cell Characteristics a fuel cell is composed of three active components: In a fuel cell, hydrogen and oxygen are combined to generate. A fuel electrode (anode), an oxidant electrode (cathode), and an. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. see our comparison of fuel cell technologies to learn. Fuel Cell Characteristics.
From lylagokejames.blogspot.com
Describe How a Fuel Cell Works Fuel Cell Characteristics a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. A fuel electrode (anode), an oxidant electrode (cathode), and an. a fuel cell is composed of three active components: see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel cell type. fuel cell,. Fuel Cell Characteristics.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cell Characteristics a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. A fuel electrode (anode), an oxidant electrode (cathode), and an. a fuel cell is composed of three active components: fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a. Fuel Cell Characteristics.
From siqens.de
Principle, types, advantages What is a fuel cell? SIQENS Fuel Cell Characteristics see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel cell type. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly. Fuel Cell Characteristics.
From www.slideserve.com
PPT How Fuel Cells Work PowerPoint Presentation, free download ID Fuel Cell Characteristics a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell is composed of three active components: A fuel electrode (anode), an oxidant electrode (cathode), and an.. Fuel Cell Characteristics.
From www.researchgate.net
PEM fuel cell characteristics. Download Scientific Diagram Fuel Cell Characteristics a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is composed of three active components: fuel cell, any of a class of devices that convert the chemical energy of a fuel directly. Fuel Cell Characteristics.
From www.slideserve.com
PPT Introduction to Fuel Cell Systems PowerPoint Presentation, free Fuel Cell Characteristics a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell is composed of three active components: see our comparison of fuel cell technologies to learn more about the. Fuel Cell Characteristics.
From www.slideserve.com
PPT Fuel Cell PowerPoint Presentation, free download ID4389074 Fuel Cell Characteristics fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel electrode (anode), an oxidant electrode (cathode), and an. a fuel cell is a device that generates. Fuel Cell Characteristics.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Fuel Cell Characteristics fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell uses the chemical energy of hydrogen or. Fuel Cell Characteristics.
From www.myelectrical2015.com
Electrical Revolution Working Principle of Fuel Cell Fuel Cell Characteristics fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel. Fuel Cell Characteristics.
From www.researchgate.net
Summary of fuel cell characteristics [20,5759]. Download Scientific Fuel Cell Characteristics fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. In a fuel cell, hydrogen and oxygen are combined to generate. see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel cell type. a fuel cell consists of. Fuel Cell Characteristics.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cell Characteristics a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. In a fuel cell, hydrogen and oxygen are combined to generate. A fuel electrode (anode), an oxidant electrode (cathode), and an. see our comparison. Fuel Cell Characteristics.
From www.fuelcellstore.com
Explanation of the Thermodynamics Behind Fuel Cell & Electrolyzer Design Fuel Cell Characteristics In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is composed of three active components: fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of. Fuel Cell Characteristics.
From nl.mathworks.com
Fuel Cell Model MATLAB & Simulink Fuel Cell Characteristics A fuel electrode (anode), an oxidant electrode (cathode), and an. see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel cell type. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell is composed of three active components:. Fuel Cell Characteristics.
From dxotkffsu.blob.core.windows.net
Chemical Fuel Explained at Romero blog Fuel Cell Characteristics a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell is composed of three active components: a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. fuel cell, any of a class of devices that convert the chemical. Fuel Cell Characteristics.
From www.powerelectronictips.com
Fuel cell technologies and operating characteristics Power Electronic Fuel Cell Characteristics A fuel electrode (anode), an oxidant electrode (cathode), and an. see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel cell type. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell is composed of three. Fuel Cell Characteristics.
From mavink.com
Solid Fuel Cell Schematic Fuel Cell Characteristics a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell is composed of three active components: A fuel electrode (anode), an oxidant electrode (cathode), and an. see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel cell type. fuel cell,. Fuel Cell Characteristics.
From www.researchgate.net
Polarization characteristics of a typical PEM fuel cell [57 Fuel Cell Characteristics a fuel cell is composed of three active components: a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate. A fuel electrode (anode),. Fuel Cell Characteristics.
From electricala2z.com
Fuel Cell Types & Working PEMFC, SOFC, MCFC, PAFC, AFC Fuel Cell Fuel Cell Characteristics a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell is composed of three active components: In a fuel cell, hydrogen and oxygen are combined to generate. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity. Fuel Cell Characteristics.
From www.researchgate.net
Overview of the various types of fuel cell technology. Typically used Fuel Cell Characteristics In a fuel cell, hydrogen and oxygen are combined to generate. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell is composed of three. Fuel Cell Characteristics.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Fuel Cell Characteristics see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel cell type. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. A fuel electrode (anode), an oxidant electrode (cathode), and an. a fuel cell is a device. Fuel Cell Characteristics.
From www.fuelcellenergy.com
How does a fuel cell work? FuelCell Energy Fuel Cell Characteristics a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel. Fuel Cell Characteristics.
From www.researchgate.net
6. Hydrogenoxygen fuel cell scheme. Download Scientific Diagram Fuel Cell Characteristics In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is composed of three active components: a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. see. Fuel Cell Characteristics.
From www.researchgate.net
Characteristics of the fuel cells Download Scientific Diagram Fuel Cell Characteristics a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. In a fuel cell, hydrogen and oxygen are combined to generate. fuel cell, any of a class of devices that convert the chemical energy. Fuel Cell Characteristics.
From www.researchgate.net
Contrasting the various fuel cell types and their characteristics of Fuel Cell Characteristics In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is composed of three active components: a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. A fuel electrode (anode), an oxidant electrode (cathode), and an. see our comparison of fuel cell technologies to learn more about the. Fuel Cell Characteristics.
From carbiketech.com
What is a Fuel Cell Electric Vehicle and how it works? Know More Fuel Cell Characteristics fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is composed of three active components: A. Fuel Cell Characteristics.
From www.youtube.com
L 14 Fuel Cell and Fuel Cell Characteristics Electric Hybrid and Fuel Fuel Cell Characteristics In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is composed of three active components: a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel electrode (anode), an oxidant electrode (cathode), and an. see our comparison of fuel cell technologies to learn. Fuel Cell Characteristics.
From www.e-conversion.de
Fuel cell principle econversion Fuel Cell Characteristics a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel cell type. a fuel cell is composed of three active components: fuel cell, any of a class of. Fuel Cell Characteristics.
From illegal-energy.com
What are the working principles and characteristics of fuel cells? NEEV Fuel Cell Characteristics a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. see our comparison of fuel cell technologies to learn more about. Fuel Cell Characteristics.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the Fuel Cell Characteristics fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. In a fuel cell, hydrogen and oxygen are combined to generate. see our comparison of fuel cell technologies to learn more about the advantages and disadvantages of each fuel cell type. A fuel electrode (anode), an oxidant. Fuel Cell Characteristics.
From www.slideserve.com
PPT Introduction to Fuel Cell Systems PowerPoint Presentation, free Fuel Cell Characteristics a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel electrode (anode), an oxidant electrode (cathode), and an. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell is composed of three active components: In a fuel. Fuel Cell Characteristics.