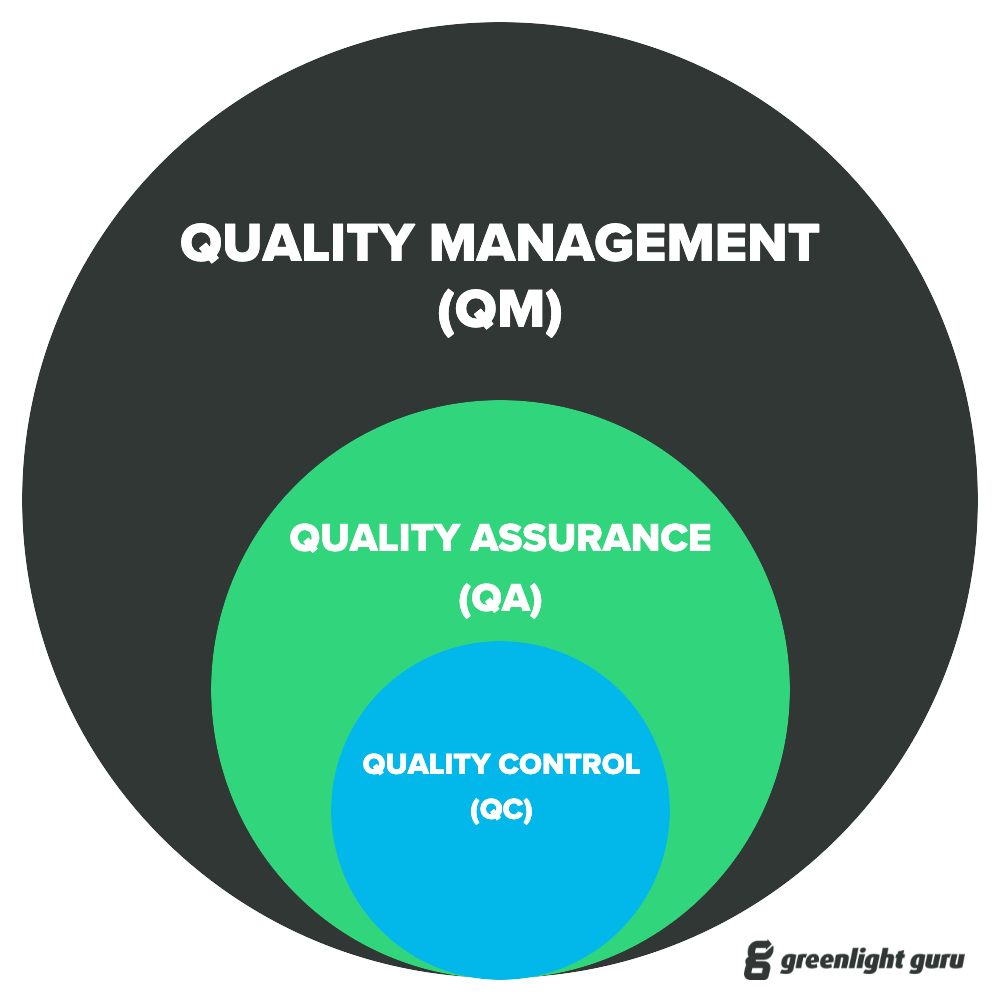
Medical Device Testing Quality Assurance . Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are being met. Validation for medical device product quality assurance. Scheduled quality assurance testing is critical to reducing patient risks, and research has shown it can cut down on maintenance costs by as much as 30%. Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. The goal is to identify and correct product flaws, eventually leading to process improvement and an overall decrease in defects. The medical device manufacturing process is monitored for quality assurance testing to ensure that all industry and regulatory standards are met. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. The purpose is to find and fix. Fda's medical device quality and compliance tools. Medical device single audit program.
from smartdataweek.com
Validation for medical device product quality assurance. Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are being met. Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. The purpose is to find and fix. Medical device single audit program. Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. Fda's medical device quality and compliance tools. The medical device manufacturing process is monitored for quality assurance testing to ensure that all industry and regulatory standards are met. The goal is to identify and correct product flaws, eventually leading to process improvement and an overall decrease in defects. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices.
Quality Assurance vs. Quality Control in the Medical Device Industry (2023)
Medical Device Testing Quality Assurance Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are being met. The medical device manufacturing process is monitored for quality assurance testing to ensure that all industry and regulatory standards are met. Scheduled quality assurance testing is critical to reducing patient risks, and research has shown it can cut down on maintenance costs by as much as 30%. Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. Validation for medical device product quality assurance. The goal is to identify and correct product flaws, eventually leading to process improvement and an overall decrease in defects. Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are being met. The purpose is to find and fix. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Medical device single audit program. Fda's medical device quality and compliance tools.
From www.youtube.com
Medical Device Quality Assurance and Regulatory Affairs Expertise 2020 Medical Device Testing Quality Assurance Validation for medical device product quality assurance. Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are being met. Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. The goal is to identify and correct product flaws, eventually leading to. Medical Device Testing Quality Assurance.
From smartdataweek.com
Quality Assurance vs. Quality Control in the Medical Device Industry (2023) Medical Device Testing Quality Assurance Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Validation for medical device product quality assurance. The medical device manufacturing process is monitored for quality assurance testing to ensure that all industry and regulatory standards are met. Medical device single audit program. The purpose is to find and fix. The goal is. Medical Device Testing Quality Assurance.
From royed.in
Quality Assurance Documentation [Pharma & Medical Device] Royed Training Medical Device Testing Quality Assurance Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. Medical device single audit program. Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. Validation and verification are inextricable from product development and process design. Medical Device Testing Quality Assurance.
From tateeda.com
Device Testing and Quality Assurance in the Healthcare Industry TATEEDA Medical Device Testing Quality Assurance Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are being met. The medical device manufacturing process is monitored for quality assurance testing to ensure that all industry and regulatory standards are met. Medical device single audit program. Validation for medical device product quality assurance. Fda's medical device quality and compliance tools. Validation and verification. Medical Device Testing Quality Assurance.
From inbels.com
QA & Testing InBels Medical Device Testing Quality Assurance Medical device single audit program. The goal is to identify and correct product flaws, eventually leading to process improvement and an overall decrease in defects. Validation for medical device product quality assurance. Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are being met. Scheduled quality assurance testing is critical to reducing patient risks, and. Medical Device Testing Quality Assurance.
From qualizeal.com
Why is Quality Assurance Important in Medical Device Testing? QualiZeal Medical Device Testing Quality Assurance Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. The medical device manufacturing process is monitored for quality assurance testing to ensure that all industry and regulatory standards are met. The purpose is to find and fix. Scheduled quality assurance testing is critical to reducing. Medical Device Testing Quality Assurance.
From greenhostit.com
Why Quality Assurance Is Essential for Medical Device Companies Green Medical Device Testing Quality Assurance The goal is to identify and correct product flaws, eventually leading to process improvement and an overall decrease in defects. Scheduled quality assurance testing is critical to reducing patient risks, and research has shown it can cut down on maintenance costs by as much as 30%. Validation and verification are inextricable from product development and process design for the manufacturing. Medical Device Testing Quality Assurance.
From megamindstechnologies.com
The Importance of Quality Assurance in Medical Device Testing Medical Device Testing Quality Assurance Validation for medical device product quality assurance. The medical device manufacturing process is monitored for quality assurance testing to ensure that all industry and regulatory standards are met. Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. Fda's medical device quality and compliance tools. Medical device single audit program.. Medical Device Testing Quality Assurance.
From pharmacyconnection.ca
Quality Assurance Enhanced Patient Through Improving Practice Medical Device Testing Quality Assurance The purpose is to find and fix. Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Fda's medical device quality and compliance tools. Medical device single audit program.. Medical Device Testing Quality Assurance.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Medical Device Testing Quality Assurance The purpose is to find and fix. Medical device single audit program. Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. The medical device manufacturing process is monitored for quality assurance testing to ensure that all industry and regulatory standards are met. The goal is to identify and correct. Medical Device Testing Quality Assurance.
From www.quality-assurance.com
ISO 13485 standard for medical devices How Effective it is? Medical Device Testing Quality Assurance The goal is to identify and correct product flaws, eventually leading to process improvement and an overall decrease in defects. The medical device manufacturing process is monitored for quality assurance testing to ensure that all industry and regulatory standards are met. Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by. Medical Device Testing Quality Assurance.
From www.slideserve.com
PPT The Importance of Quality Assurance in Medical Device Testing Medical Device Testing Quality Assurance Scheduled quality assurance testing is critical to reducing patient risks, and research has shown it can cut down on maintenance costs by as much as 30%. Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. Medical device qa is a framework supported by systematic approaches. Medical Device Testing Quality Assurance.
From igmpi.ac.in
Quality Assurance and Quality Control (Medical Device as Major) Medical Device Testing Quality Assurance Validation for medical device product quality assurance. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Medical device single audit program. The purpose is to find and fix. Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. Scheduled quality assurance testing. Medical Device Testing Quality Assurance.
From iconic.com.bd
Importance of Quality Assurance and Quality Control in Medical Device Medical Device Testing Quality Assurance The goal is to identify and correct product flaws, eventually leading to process improvement and an overall decrease in defects. Fda's medical device quality and compliance tools. Medical device single audit program. Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. Validation for medical device product quality assurance. Validation. Medical Device Testing Quality Assurance.
From huddle.eurostarsoftwaretesting.com
Why QA is Critical for Medical Device Testing EuroSTAR Huddle Medical Device Testing Quality Assurance Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. The medical device manufacturing process is monitored for quality assurance testing to ensure that all industry and regulatory standards. Medical Device Testing Quality Assurance.
From www.tuleap.org
ISO 13485 Why implement a Quality Management System (QMS) for medical Medical Device Testing Quality Assurance Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. The medical device manufacturing process is monitored for quality assurance testing to ensure that all industry and regulatory standards are met. Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are. Medical Device Testing Quality Assurance.
From www.orielstat.com
What is a Medical Device Quality Management System (QMS)? Medical Device Testing Quality Assurance Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. The purpose is to find and fix. The goal is to identify and correct product flaws, eventually leading to process improvement and an overall decrease in defects. Medical device quality assurance (qa) refers to the systematic and comprehensive set of. Medical Device Testing Quality Assurance.
From healthcareknowledgesathi.blogspot.com
Importance of Quality Assurance Programme for Pathology Department Medical Device Testing Quality Assurance Scheduled quality assurance testing is critical to reducing patient risks, and research has shown it can cut down on maintenance costs by as much as 30%. Medical device single audit program. Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. Validation and verification are inextricable. Medical Device Testing Quality Assurance.
From smartdataweek.com
Quality Assurance vs. Quality Control in the Medical Device Industry (2023) Medical Device Testing Quality Assurance Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are being met. Scheduled quality assurance testing is critical to reducing patient risks, and research has shown it can cut down on maintenance costs by as much as 30%. The goal is to identify and correct product flaws, eventually leading to process improvement and an overall. Medical Device Testing Quality Assurance.
From www.youtube.com
Medical Device Quality Assurance Testing Best Practices For Patient Medical Device Testing Quality Assurance Scheduled quality assurance testing is critical to reducing patient risks, and research has shown it can cut down on maintenance costs by as much as 30%. Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. The goal is to identify and correct product flaws, eventually. Medical Device Testing Quality Assurance.
From www.joharidigital.com
Medical Device Testing Types, Procedures, and Best Practices Medical Device Testing Quality Assurance Validation for medical device product quality assurance. Scheduled quality assurance testing is critical to reducing patient risks, and research has shown it can cut down on maintenance costs by as much as 30%. Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are being met. The purpose is to find and fix. Medical device quality. Medical Device Testing Quality Assurance.
From blog.qasource.com
Why is Quality Assurance and Quality Control Important in Medical Medical Device Testing Quality Assurance The goal is to identify and correct product flaws, eventually leading to process improvement and an overall decrease in defects. Scheduled quality assurance testing is critical to reducing patient risks, and research has shown it can cut down on maintenance costs by as much as 30%. The medical device manufacturing process is monitored for quality assurance testing to ensure that. Medical Device Testing Quality Assurance.
From www.greenlight.guru
Quality Assurance vs. Quality Control in the Medical Device Industry Medical Device Testing Quality Assurance Scheduled quality assurance testing is critical to reducing patient risks, and research has shown it can cut down on maintenance costs by as much as 30%. Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. The medical device manufacturing process is monitored for quality assurance testing to ensure that. Medical Device Testing Quality Assurance.
From www.impactqa.com
Why QA & QC are an essential part of Medical Device Testing? Medical Device Testing Quality Assurance Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. Validation for medical device product quality assurance. Medical device single audit program. Validation and verification are. Medical Device Testing Quality Assurance.
From iteratorstesting.com
How To Measure Quality Assurance Performance With KPIs and Metrics For Medical Device Testing Quality Assurance Medical device single audit program. Fda's medical device quality and compliance tools. Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are being met. The medical device manufacturing process is monitored. Medical Device Testing Quality Assurance.
From iziel.com
QMS Documentation for Medical Devices ISO 13485 Certification IZiel Medical Device Testing Quality Assurance Scheduled quality assurance testing is critical to reducing patient risks, and research has shown it can cut down on maintenance costs by as much as 30%. Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are being met. Fda's medical device quality and compliance tools. The purpose is to find and fix. The medical device. Medical Device Testing Quality Assurance.
From www.slideserve.com
PPT Medical Device Quality Enhancement An Industry Perspective Medical Device Testing Quality Assurance Scheduled quality assurance testing is critical to reducing patient risks, and research has shown it can cut down on maintenance costs by as much as 30%. The purpose is to find and fix. Medical device single audit program. Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. Fda's medical. Medical Device Testing Quality Assurance.
From www.slideserve.com
PPT Medical Device Software Quality Assurance and Risk Assessment Medical Device Testing Quality Assurance Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Validation for medical device product quality assurance. Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. The purpose is to find and fix. Quality assurance testing monitors the medical device manufacturing process. Medical Device Testing Quality Assurance.
From www.dotcompliance.com
Top 10 Medical Device Testing Companies of 2021 Dot Compliance Medical Device Testing Quality Assurance Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. Fda's medical device quality and compliance tools. Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are being met. The medical device manufacturing process is monitored for quality assurance testing to. Medical Device Testing Quality Assurance.
From www.joharidigital.com
Importance of Quality Management System in Medical Device Manufacturing Medical Device Testing Quality Assurance Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. The purpose is to find and fix. The goal is to identify and correct product flaws, eventually leading to process improvement and an overall decrease in defects. The medical device manufacturing process is monitored for quality assurance testing to ensure. Medical Device Testing Quality Assurance.
From qualizeal.com
Why is Quality Assurance Important in Medical Device Testing? QualiZeal Medical Device Testing Quality Assurance Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are being met. Medical device single audit program. Fda's. Medical Device Testing Quality Assurance.
From www.impactqa.com
Why QA & QC are an essential part of Medical Device Testing? Medical Device Testing Quality Assurance The goal is to identify and correct product flaws, eventually leading to process improvement and an overall decrease in defects. Validation for medical device product quality assurance. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. The medical device manufacturing process is monitored for quality assurance testing to ensure that all industry. Medical Device Testing Quality Assurance.
From www.slideteam.net
Pharmaceutical Quality Control And Assurance Process PPT Presentation Medical Device Testing Quality Assurance Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. Fda's medical device quality and compliance tools. Medical device quality assurance (qa) refers to the systematic and comprehensive set of processes, procedures, and activities implemented by manufacturers and regulatory bodies to. The purpose is to find and fix. Quality assurance. Medical Device Testing Quality Assurance.
From www.joharidigital.com
Importance of Quality Management System in Medical Device Manufacturing Medical Device Testing Quality Assurance Medical device single audit program. Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Scheduled quality assurance testing is critical to reducing patient risks, and research has shown it can cut down. Medical Device Testing Quality Assurance.
From elementtechnologies.net
Quality Assurance and other Services Life Sciences Services Element Medical Device Testing Quality Assurance Fda's medical device quality and compliance tools. Medical device single audit program. The purpose is to find and fix. Medical device qa is a framework supported by systematic approaches that ensure the uniform and high quality of medical devices. Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are being met. Validation for medical device. Medical Device Testing Quality Assurance.