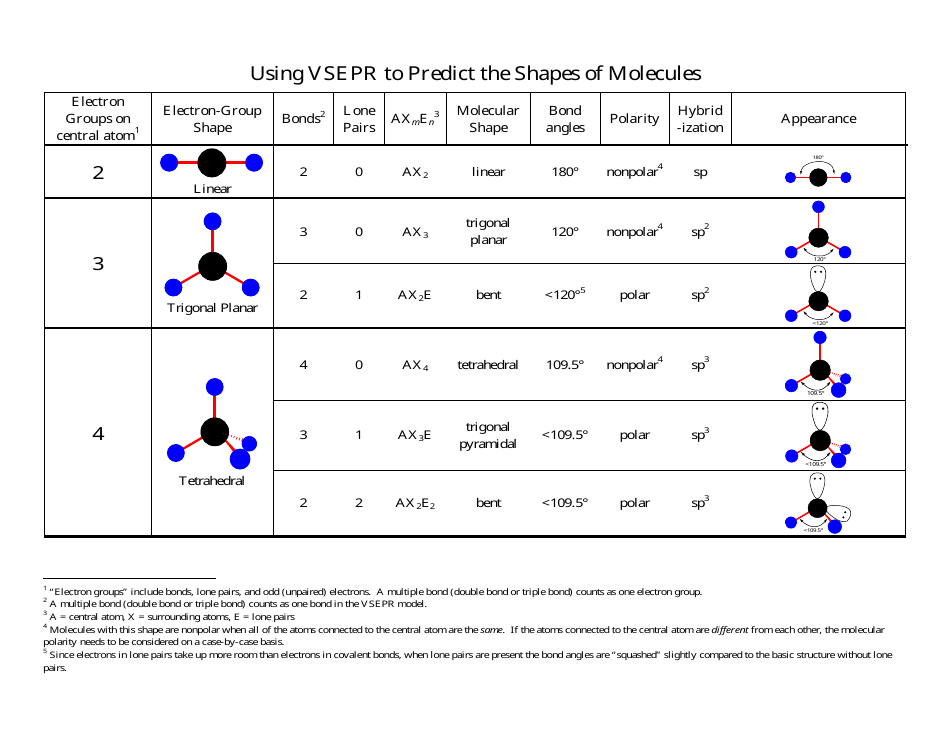
Formaldehyde Vsepr Shape . Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). It basically says that electron pairs,. The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize the overall formal. Using the vsepr theory, it can be seen that ch 2 o represents the chemical formula of ax3. The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as.
from www.templateroller.com
The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. It basically says that electron pairs,. Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize the overall formal. Using the vsepr theory, it can be seen that ch 2 o represents the chemical formula of ax3.
Vsepr and the Shapes of Molecules Chart Download Printable PDF
Formaldehyde Vsepr Shape Using the vsepr theory, it can be seen that ch 2 o represents the chemical formula of ax3. Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). Using the vsepr theory, it can be seen that ch 2 o represents the chemical formula of ax3. The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize the overall formal. It basically says that electron pairs,.
From nl.pinterest.com
What is the molecular geometry of "H"_2"O"? Draw its VSEPR structure Formaldehyde Vsepr Shape The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. Using the vsepr theory, it can be seen that ch 2 o represents the chemical formula of ax3.. Formaldehyde Vsepr Shape.
From www.templateroller.com
Vsepr Theory (Molecular Shapes) Chart Download Printable PDF Formaldehyde Vsepr Shape Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize the overall formal. Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of. Formaldehyde Vsepr Shape.
From courses.lumenlearning.com
Molecular Geometry Boundless Chemistry Formaldehyde Vsepr Shape The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize the overall formal. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). It basically says that electron pairs,. Using the vsepr theory, it can be seen that ch 2 o represents the chemical. Formaldehyde Vsepr Shape.
From mungfali.com
VSEPR Geometry Chart Formaldehyde Vsepr Shape Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. It basically says that electron pairs,. The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. Formaldehyde depicted by a lewis structure (left), ball and stick model (center),. Formaldehyde Vsepr Shape.
From www.youtube.com
The shape of formaldehyde molecule as per the VSEPR theory is a Formaldehyde Vsepr Shape Using the vsepr theory, it can be seen that ch 2 o represents the chemical formula of ax3. The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right).. Formaldehyde Vsepr Shape.
From jsmithmoore.com
Vsepr theory chart Formaldehyde Vsepr Shape It basically says that electron pairs,. Using the vsepr theory, it can be seen that ch 2 o represents the chemical formula of ax3. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). Once we know. Formaldehyde Vsepr Shape.
From testbook.com
VSEPR Theory Definition, Postulates, Formula and Examples Formaldehyde Vsepr Shape It basically says that electron pairs,. The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and. Formaldehyde Vsepr Shape.
From mavink.com
Theory Vsepr Lewis Structure Formaldehyde Vsepr Shape Using the vsepr theory, it can be seen that ch 2 o represents the chemical formula of ax3. The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize the overall formal. Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and. Formaldehyde Vsepr Shape.
From www.slideserve.com
PPT Molecular Geometry and VSEPR Theory PowerPoint Presentation, free Formaldehyde Vsepr Shape Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). Using the vsepr theory, it can be seen that ch 2 o represents the chemical formula of ax3. The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as.. Formaldehyde Vsepr Shape.
From www.chemistrylearner.com
VSEPR Theory Explanation, Chart, and Examples Formaldehyde Vsepr Shape Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. It basically says that electron pairs,. Formaldehyde depicted by a lewis structure (left), ball and stick model (center),. Formaldehyde Vsepr Shape.
From mavink.com
Vsepr Theory Table Formaldehyde Vsepr Shape Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. It basically says that electron pairs,. Using the vsepr theory, it can be seen that ch 2 o represents the chemical formula of ax3. The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and. Formaldehyde Vsepr Shape.
From www.animalia-life.club
Formaldehyde Molecular Geometry Formaldehyde Vsepr Shape The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. It basically says that electron pairs,. Using the vsepr theory, it can be seen that ch 2 o represents the chemical formula of ax3. The basic idea in molecular shapes is called valence shell electron pair repulsion. Formaldehyde Vsepr Shape.
From www.coursehero.com
[Solved] Lewis Structures and VSEPR formaldehyde and thioformaldehyde Formaldehyde Vsepr Shape It basically says that electron pairs,. The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize the overall formal. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). The vsepr model states that the electron regions around an atom spread out to make. Formaldehyde Vsepr Shape.
From chem.libretexts.org
2.1 Valence Bond Theory Chemistry LibreTexts Formaldehyde Vsepr Shape Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. The vsepr model states that the electron regions around an atom spread out to make each one as far from the others. Formaldehyde Vsepr Shape.
From www.thoughtco.com
VSEPR Theory Definition in Chemistry Formaldehyde Vsepr Shape It basically says that electron pairs,. Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). Using the. Formaldehyde Vsepr Shape.
From jsmithmoore.com
Vsepr theory chart Formaldehyde Vsepr Shape It basically says that electron pairs,. Using the vsepr theory, it can be seen that ch 2 o represents the chemical formula of ax3. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. The lewis structure. Formaldehyde Vsepr Shape.
From www.aakash.ac.in
VSEPR Theory Postulates, Prediction of geometrical shapes, Limitations Formaldehyde Vsepr Shape The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize the overall formal. It basically says that electron pairs,. The basic idea in molecular shapes. Formaldehyde Vsepr Shape.
From study.com
VSEPR Theory Chart & Model Lesson Formaldehyde Vsepr Shape The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). Using the vsepr theory,. Formaldehyde Vsepr Shape.
From www.scribd.com
VSEPR / Molecular Geometry Chart Formaldehyde Vsepr Shape The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize the overall formal. It basically says that electron pairs,. Once we know the lewis dot. Formaldehyde Vsepr Shape.
From general.chemistrysteps.com
VSEPR Theory Chemistry Steps Formaldehyde Vsepr Shape The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize the overall formal. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. It basically. Formaldehyde Vsepr Shape.
From www.slideserve.com
PPT Chemical Bonding and Molecular Structure (Chapter 9) PowerPoint Formaldehyde Vsepr Shape It basically says that electron pairs,. Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. The vsepr. Formaldehyde Vsepr Shape.
From mavink.com
Vsepr Summary Chart Formaldehyde Vsepr Shape It basically says that electron pairs,. Using the vsepr theory, it can be seen that ch 2 o represents the chemical formula of ax3. The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and. Formaldehyde Vsepr Shape.
From www.animalia-life.club
Formaldehyde Molecular Geometry Formaldehyde Vsepr Shape It basically says that electron pairs,. Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely. Formaldehyde Vsepr Shape.
From chem.libretexts.org
VSEPR Chemistry LibreTexts Formaldehyde Vsepr Shape Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize the overall formal. The vsepr. Formaldehyde Vsepr Shape.
From scienceglen.com
VSEPR Kit Science Glen Formaldehyde Vsepr Shape It basically says that electron pairs,. The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. Using the vsepr theory, it can be seen that ch 2 o represents the chemical formula of ax3. Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and. Formaldehyde Vsepr Shape.
From eechem2.net
eeCHEM handout summary of VSEPR Model Formaldehyde Vsepr Shape The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen. Formaldehyde Vsepr Shape.
From brunofuga.adv.br
VSEPR Theory Explanation, Chart, And Examples, 51 OFF Formaldehyde Vsepr Shape The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize the overall formal. It basically. Formaldehyde Vsepr Shape.
From chemsjc.blogspot.com
Jimchem VSEPR Theory Formaldehyde Vsepr Shape The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. It basically says that electron pairs,. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and. Formaldehyde Vsepr Shape.
From www.chegg.com
Solved Question 5) The VSEPR shape of formaldehyde is shown Formaldehyde Vsepr Shape The vsepr model states that the electron regions around an atom spread out to make each one as far from the others as. Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. It basically says that electron pairs,. The lewis structure of formaldehyde (ch2o) shows how electrons are being shared. Formaldehyde Vsepr Shape.
From www.vrogue.co
Molecular Shapes Vsepr Theory vrogue.co Formaldehyde Vsepr Shape The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). The vsepr model states that the electron regions. Formaldehyde Vsepr Shape.
From www.templateroller.com
Vsepr and the Shapes of Molecules Chart Download Printable PDF Formaldehyde Vsepr Shape Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). The vsepr model states that the electron regions. Formaldehyde Vsepr Shape.
From mungfali.com
VSEPR Theory Table Formaldehyde Vsepr Shape It basically says that electron pairs,. Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize the overall formal. The vsepr model states that the electron regions around an. Formaldehyde Vsepr Shape.
From chem.libretexts.org
10.2 VSEPR Theory The Five Basic Shapes Chemistry LibreTexts Formaldehyde Vsepr Shape Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization and molecular geometry. The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize. Formaldehyde Vsepr Shape.
From mungfali.com
VSEPR Theory Table Formaldehyde Vsepr Shape The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize the overall formal. Using the vsepr theory, it can be seen that ch 2 o represents the chemical formula of ax3. The basic idea in molecular shapes is called valence shell electron pair repulsion (vsepr). Formaldehyde depicted by. Formaldehyde Vsepr Shape.
From mavink.com
Vsepr Electron Geometry Formaldehyde Vsepr Shape The lewis structure of formaldehyde (ch2o) shows how electrons are being shared among the carbon, oxygen, and hydrogen atoms to completely neutralize the overall formal. Formaldehyde depicted by a lewis structure (left), ball and stick model (center), and a comparison of bond lengths (right). Once we know the lewis dot structure of formaldehyde, we can easily find out its hybridization. Formaldehyde Vsepr Shape.