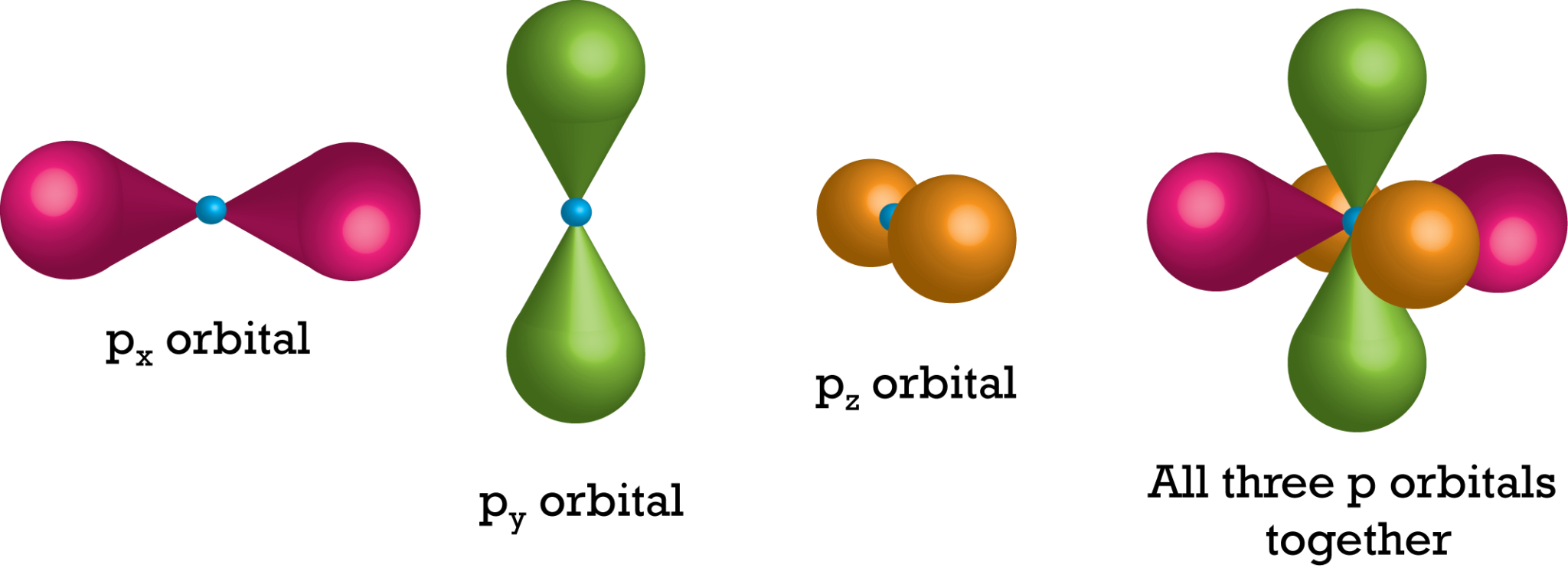
What Is The Orbital Shell . Shell is the pathway followed by electrons around an atom’s nucleus. Each subshell can hold a specific number of orbitals: ℓ ℓ, the orbital angular momentum quantum number defines the subshell. Each shell can be divided further into subshells, labelled s, p, d and f; 2) orbitals are combined when bonds form between atoms in a molecule. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and. Orbitals within a shell are divided into subshells. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for. This quantum number defines the shape of the orbitals (probability densities) that the electrons. Subshell is the pathway in which an. Orbitals that have the same value of the principal quantum number $n$ form a shell.
from chemistryskills.com
2) orbitals are combined when bonds form between atoms in a molecule. Orbitals within a shell are divided into subshells. Orbitals that have the same value of the principal quantum number $n$ form a shell. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and. Each shell can be divided further into subshells, labelled s, p, d and f; Shell is the pathway followed by electrons around an atom’s nucleus. Subshell is the pathway in which an. This quantum number defines the shape of the orbitals (probability densities) that the electrons. ℓ ℓ, the orbital angular momentum quantum number defines the subshell. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for.
Shapes of Orbitals and their Types Chemistry Skills
What Is The Orbital Shell This quantum number defines the shape of the orbitals (probability densities) that the electrons. 2) orbitals are combined when bonds form between atoms in a molecule. ℓ ℓ, the orbital angular momentum quantum number defines the subshell. Each subshell can hold a specific number of orbitals: Shell is the pathway followed by electrons around an atom’s nucleus. Orbitals that have the same value of the principal quantum number $n$ form a shell. Each shell can be divided further into subshells, labelled s, p, d and f; Subshell is the pathway in which an. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for. Orbitals within a shell are divided into subshells. This quantum number defines the shape of the orbitals (probability densities) that the electrons. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics What Is The Orbital Shell This quantum number defines the shape of the orbitals (probability densities) that the electrons. Subshell is the pathway in which an. Each shell can be divided further into subshells, labelled s, p, d and f; Shell is the pathway followed by electrons around an atom’s nucleus. ℓ ℓ, the orbital angular momentum quantum number defines the subshell. There are four. What Is The Orbital Shell.
From www.youtube.com
Shells, Subshells, and Orbitals l Understand the difference YouTube What Is The Orbital Shell Orbitals that have the same value of the principal quantum number $n$ form a shell. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for. Each shell can be divided further into subshells, labelled s, p, d and f; There are four. What Is The Orbital Shell.
From byjus.com
what is the sequence of shell , subshell, orbitals , orbits , electrons What Is The Orbital Shell Subshell is the pathway in which an. Orbitals within a shell are divided into subshells. Shell is the pathway followed by electrons around an atom’s nucleus. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for. This quantum number defines the shape. What Is The Orbital Shell.
From diagrambomenslikpo.z13.web.core.windows.net
Understanding Molecular Orbital Diagrams What Is The Orbital Shell 2) orbitals are combined when bonds form between atoms in a molecule. Each shell can be divided further into subshells, labelled s, p, d and f; Orbitals that have the same value of the principal quantum number $n$ form a shell. Each subshell can hold a specific number of orbitals: Subshell is the pathway in which an. There are four. What Is The Orbital Shell.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is The Orbital Shell ℓ ℓ, the orbital angular momentum quantum number defines the subshell. Orbitals that have the same value of the principal quantum number $n$ form a shell. Subshell is the pathway in which an. 2) orbitals are combined when bonds form between atoms in a molecule. Each shell can be divided further into subshells, labelled s, p, d and f; There. What Is The Orbital Shell.
From socratic.org
What is the difference between electron shells and electron orbitals What Is The Orbital Shell The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for. Each subshell can hold a specific number of orbitals: 2) orbitals are combined when bonds form between atoms in a molecule. Subshell is the pathway in which an. This quantum number defines. What Is The Orbital Shell.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica What Is The Orbital Shell 2) orbitals are combined when bonds form between atoms in a molecule. Shell is the pathway followed by electrons around an atom’s nucleus. Subshell is the pathway in which an. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for. Orbitals that. What Is The Orbital Shell.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills What Is The Orbital Shell Orbitals within a shell are divided into subshells. ℓ ℓ, the orbital angular momentum quantum number defines the subshell. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and. Subshell is the pathway in which an. Each shell can be divided further into subshells, labelled s, p, d and. What Is The Orbital Shell.
From socratic.org
What is the difference between Shell (orbit) , Subshell and orbital What Is The Orbital Shell Orbitals within a shell are divided into subshells. Shell is the pathway followed by electrons around an atom’s nucleus. ℓ ℓ, the orbital angular momentum quantum number defines the subshell. Each shell can be divided further into subshells, labelled s, p, d and f; There are four types of orbitals that you should be familiar with s, p, d and. What Is The Orbital Shell.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements What Is The Orbital Shell Shell is the pathway followed by electrons around an atom’s nucleus. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for.. What Is The Orbital Shell.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Is The Orbital Shell Shell is the pathway followed by electrons around an atom’s nucleus. This quantum number defines the shape of the orbitals (probability densities) that the electrons. Each shell can be divided further into subshells, labelled s, p, d and f; Orbitals that have the same value of the principal quantum number $n$ form a shell. Subshell is the pathway in which. What Is The Orbital Shell.
From www.vedantu.com
Define an atomic orbital. What Is The Orbital Shell Each shell can be divided further into subshells, labelled s, p, d and f; Shell is the pathway followed by electrons around an atom’s nucleus. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for. 2) orbitals are combined when bonds form. What Is The Orbital Shell.
From courses.lumenlearning.com
Atoms and Elements Biology for Majors I What Is The Orbital Shell Orbitals within a shell are divided into subshells. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for. 2) orbitals are combined when bonds form between atoms in a molecule. ℓ ℓ, the orbital angular momentum quantum number defines the subshell. Each. What Is The Orbital Shell.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free What Is The Orbital Shell Shell is the pathway followed by electrons around an atom’s nucleus. Each shell can be divided further into subshells, labelled s, p, d and f; This quantum number defines the shape of the orbitals (probability densities) that the electrons. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s. What Is The Orbital Shell.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Is The Orbital Shell 2) orbitals are combined when bonds form between atoms in a molecule. Each shell can be divided further into subshells, labelled s, p, d and f; There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and. ℓ ℓ, the orbital angular momentum quantum number defines the subshell. Shell is. What Is The Orbital Shell.
From www.slideserve.com
PPT 1. Structure and Bonding PowerPoint Presentation, free download What Is The Orbital Shell Each shell can be divided further into subshells, labelled s, p, d and f; Shell is the pathway followed by electrons around an atom’s nucleus. 2) orbitals are combined when bonds form between atoms in a molecule. ℓ ℓ, the orbital angular momentum quantum number defines the subshell. Orbitals that have the same value of the principal quantum number $n$. What Is The Orbital Shell.
From gzscienceclassonline.weebly.com
1. Electron Configuration What Is The Orbital Shell There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and. Subshell is the pathway in which an. ℓ ℓ, the orbital angular momentum quantum number defines the subshell. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s. What Is The Orbital Shell.
From www.vedantu.com
Distribution of Electrons in Different Orbits/Shells Learn Important What Is The Orbital Shell Orbitals that have the same value of the principal quantum number $n$ form a shell. Orbitals within a shell are divided into subshells. 2) orbitals are combined when bonds form between atoms in a molecule. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and. ℓ ℓ, the orbital. What Is The Orbital Shell.
From pnghut.com
Electron Configuration Atomic Orbital Shell Energy Level Iron What Is The Orbital Shell Subshell is the pathway in which an. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for. Each shell can be. What Is The Orbital Shell.
From www.thoughtco.com
Electron Configuration Chart What Is The Orbital Shell 2) orbitals are combined when bonds form between atoms in a molecule. Shell is the pathway followed by electrons around an atom’s nucleus. Each subshell can hold a specific number of orbitals: ℓ ℓ, the orbital angular momentum quantum number defines the subshell. Each shell can be divided further into subshells, labelled s, p, d and f; Subshell is the. What Is The Orbital Shell.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Is The Orbital Shell Each subshell can hold a specific number of orbitals: Orbitals that have the same value of the principal quantum number $n$ form a shell. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and. Shell is the pathway followed by electrons around an atom’s nucleus. Orbitals within a shell. What Is The Orbital Shell.
From www.britannica.com
Electron shell Definition & Facts Britannica What Is The Orbital Shell There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and. Orbitals that have the same value of the principal quantum number $n$ form a shell. 2) orbitals are combined when bonds form between atoms in a molecule. Each subshell can hold a specific number of orbitals: This quantum number. What Is The Orbital Shell.
From www.britannica.com
Orbital Chemistry, Physics & Applications Britannica What Is The Orbital Shell Each subshell can hold a specific number of orbitals: 2) orbitals are combined when bonds form between atoms in a molecule. Each shell can be divided further into subshells, labelled s, p, d and f; Orbitals within a shell are divided into subshells. Subshell is the pathway in which an. This quantum number defines the shape of the orbitals (probability. What Is The Orbital Shell.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent What Is The Orbital Shell Shell is the pathway followed by electrons around an atom’s nucleus. Each subshell can hold a specific number of orbitals: Subshell is the pathway in which an. Each shell can be divided further into subshells, labelled s, p, d and f; Orbitals that have the same value of the principal quantum number $n$ form a shell. This quantum number defines. What Is The Orbital Shell.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell What Is The Orbital Shell The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and. 2) orbitals are combined when bonds form between atoms in a. What Is The Orbital Shell.
From pediaa.com
Difference Between Shell Subshell and Orbital Definition, Structure What Is The Orbital Shell Subshell is the pathway in which an. Each subshell can hold a specific number of orbitals: There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and. Orbitals that have the same value of the principal quantum number $n$ form a shell. Orbitals within a shell are divided into subshells.. What Is The Orbital Shell.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills What Is The Orbital Shell Each subshell can hold a specific number of orbitals: Each shell can be divided further into subshells, labelled s, p, d and f; There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and. The letter in the orbital name defines the subshell with a specific angular momentum quantum number. What Is The Orbital Shell.
From bilifforyou.blogspot.com
Why are the orbitals shells called s, p, d, f, etc.? Is there a reason What Is The Orbital Shell Shell is the pathway followed by electrons around an atom’s nucleus. Each subshell can hold a specific number of orbitals: This quantum number defines the shape of the orbitals (probability densities) that the electrons. Subshell is the pathway in which an. Each shell can be divided further into subshells, labelled s, p, d and f; Orbitals that have the same. What Is The Orbital Shell.
From antranik.org
Electrons shells and orbitals What Is The Orbital Shell The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for. Subshell is the pathway in which an. This quantum number defines the shape of the orbitals (probability densities) that the electrons. Orbitals that have the same value of the principal quantum number. What Is The Orbital Shell.
From mungfali.com
What Is An Electron Shell What Is The Orbital Shell ℓ ℓ, the orbital angular momentum quantum number defines the subshell. Subshell is the pathway in which an. Orbitals that have the same value of the principal quantum number $n$ form a shell. Each subshell can hold a specific number of orbitals: This quantum number defines the shape of the orbitals (probability densities) that the electrons. 2) orbitals are combined. What Is The Orbital Shell.
From www.britannica.com
Chemical bonding Atomic Orbitals, Shapes, Hybridization Britannica What Is The Orbital Shell 2) orbitals are combined when bonds form between atoms in a molecule. Subshell is the pathway in which an. Shell is the pathway followed by electrons around an atom’s nucleus. Orbitals that have the same value of the principal quantum number $n$ form a shell. There are four types of orbitals that you should be familiar with s, p, d. What Is The Orbital Shell.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho What Is The Orbital Shell The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for. This quantum number defines the shape of the orbitals (probability densities) that the electrons. 2) orbitals are combined when bonds form between atoms in a molecule. Each shell can be divided further. What Is The Orbital Shell.
From www.pinterest.com
Atoms, shells ,Subshells and Orbitals Atom, Submarine, Shells What Is The Orbital Shell Each shell can be divided further into subshells, labelled s, p, d and f; Orbitals that have the same value of the principal quantum number $n$ form a shell. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for. This quantum number. What Is The Orbital Shell.
From icdsc.org
How many electrons are in each shell including 3p orbitals What Is The Orbital Shell 2) orbitals are combined when bonds form between atoms in a molecule. This quantum number defines the shape of the orbitals (probability densities) that the electrons. ℓ ℓ, the orbital angular momentum quantum number defines the subshell. Each shell can be divided further into subshells, labelled s, p, d and f; Each subshell can hold a specific number of orbitals:. What Is The Orbital Shell.
From acemichael888.weebly.com
Electron Arrangement in Atoms Elements and the Periodic Table What Is The Orbital Shell Each subshell can hold a specific number of orbitals: 2) orbitals are combined when bonds form between atoms in a molecule. Subshell is the pathway in which an. ℓ ℓ, the orbital angular momentum quantum number defines the subshell. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s. What Is The Orbital Shell.