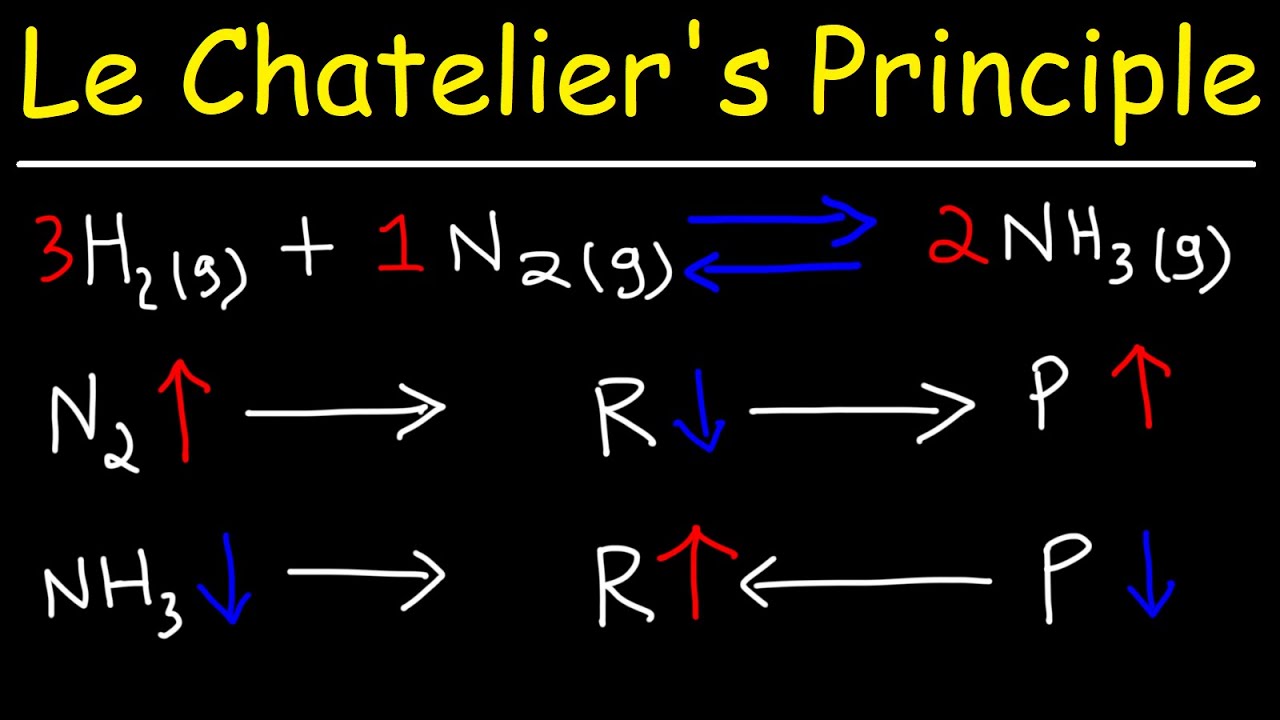
Catalyst Le Chatelier . Catalysts have sneaked onto this page under false pretences, because adding a. although low temperatures favor product formation for this exothermic process, the reaction rate at low temperatures is. Adding a catalyst does not shift the equilibrium of a chemical reaction because it. le chatelier's principle and catalysts. However, a catalyst does not affect the extent or position of a. this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. le chatelier’s principle does not apply to catalysts. overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds.
from www.youtube.com
overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. Catalysts have sneaked onto this page under false pretences, because adding a. Adding a catalyst does not shift the equilibrium of a chemical reaction because it. le chatelier's principle and catalysts. this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. le chatelier’s principle does not apply to catalysts. However, a catalyst does not affect the extent or position of a. although low temperatures favor product formation for this exothermic process, the reaction rate at low temperatures is. this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic.
Le Chatelier's Principle YouTube
Catalyst Le Chatelier Catalysts have sneaked onto this page under false pretences, because adding a. although low temperatures favor product formation for this exothermic process, the reaction rate at low temperatures is. this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. Catalysts have sneaked onto this page under false pretences, because adding a. le chatelier’s principle does not apply to catalysts. overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. However, a catalyst does not affect the extent or position of a. Adding a catalyst does not shift the equilibrium of a chemical reaction because it. this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. le chatelier's principle and catalysts.
From www.youtube.com
Le Chatelier's Principle Addition of a Catalyst YouTube Catalyst Le Chatelier this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. le chatelier’s principle does not apply to catalysts. overall, a catalyst is not a reactant and. Catalyst Le Chatelier.
From www.slideserve.com
PPT Le Chatelier’s Principle PowerPoint Presentation, free download Catalyst Le Chatelier However, a catalyst does not affect the extent or position of a. this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. Adding a catalyst does not shift the equilibrium of a chemical reaction because it. although low temperatures favor product formation for this exothermic process, the reaction. Catalyst Le Chatelier.
From www.youtube.com
Le Chatelier's Principle Volume/Pressure, Catalyst, Inert gas Catalyst Le Chatelier However, a catalyst does not affect the extent or position of a. this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. Adding a catalyst does not shift the equilibrium of a chemical reaction because it. le chatelier’s principle does not apply to catalysts. le chatelier's principle. Catalyst Le Chatelier.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Catalyst Le Chatelier le chatelier's principle and catalysts. this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. However, a catalyst does not affect the extent or position of a. although low temperatures favor product formation for this exothermic process, the reaction rate at low temperatures is. le chatelier’s. Catalyst Le Chatelier.
From readchemistry.com
Le Chatelier’s principle Read Chemistry Catalyst Le Chatelier le chatelier's principle and catalysts. this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. le chatelier’s principle does not apply to catalysts. overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. However, a. Catalyst Le Chatelier.
From www.youtube.com
Le Chatelier's Principle Effect of Catalyst SDS SK015 Topic 6.3 Catalyst Le Chatelier le chatelier’s principle does not apply to catalysts. this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. However, a catalyst does not affect the extent or position of a. Catalysts have sneaked onto this page under false pretences, because adding a. Adding a catalyst does not shift. Catalyst Le Chatelier.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Catalyst Le Chatelier Adding a catalyst does not shift the equilibrium of a chemical reaction because it. this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. le chatelier's principle. Catalyst Le Chatelier.
From slideplayer.com
Chemical Equilibrium Collision theory Rates of reactions ppt download Catalyst Le Chatelier Adding a catalyst does not shift the equilibrium of a chemical reaction because it. le chatelier’s principle does not apply to catalysts. Catalysts have sneaked onto this page under false pretences, because adding a. although low temperatures favor product formation for this exothermic process, the reaction rate at low temperatures is. However, a catalyst does not affect the. Catalyst Le Chatelier.
From screenpal.com
Lecture B2 4 Le chatelier's Principle Catalysts Catalyst Le Chatelier le chatelier's principle and catalysts. this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. However, a catalyst does not affect the extent or position of a. this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic.. Catalyst Le Chatelier.
From slideplayer.com
Le Chatelier’s Principle and Equilibrium ppt download Catalyst Le Chatelier Catalysts have sneaked onto this page under false pretences, because adding a. le chatelier's principle and catalysts. although low temperatures favor product formation for this exothermic process, the reaction rate at low temperatures is. overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. this. Catalyst Le Chatelier.
From www.youtube.com
LeChatelier's Principle Adding a catalyst Equilibrium Chemistry Catalyst Le Chatelier this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. le chatelier’s principle does not apply to catalysts. overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. Adding a catalyst does not shift the equilibrium. Catalyst Le Chatelier.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID1752058 Catalyst Le Chatelier Catalysts have sneaked onto this page under false pretences, because adding a. Adding a catalyst does not shift the equilibrium of a chemical reaction because it. le chatelier's principle and catalysts. this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. le chatelier’s principle does not apply. Catalyst Le Chatelier.
From www.youtube.com
Le Chatelier's Principle (Temperature & Catalyst) Topic 6 SK015 YouTube Catalyst Le Chatelier Catalysts have sneaked onto this page under false pretences, because adding a. this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. although low temperatures favor product formation for this exothermic process, the reaction rate at low temperatures is. Adding a catalyst does not shift the equilibrium of. Catalyst Le Chatelier.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID5648517 Catalyst Le Chatelier overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. le chatelier's principle and catalysts. Catalysts have sneaked onto this page under false pretences, because adding a. Adding a catalyst does not shift the equilibrium of a chemical reaction because it. although low temperatures favor product. Catalyst Le Chatelier.
From www.slideshare.net
Tang 02 le châtelier’s principle 2 Catalyst Le Chatelier However, a catalyst does not affect the extent or position of a. le chatelier’s principle does not apply to catalysts. although low temperatures favor product formation for this exothermic process, the reaction rate at low temperatures is. le chatelier's principle and catalysts. this page looks at le châtelier's principle and explains how to apply it to. Catalyst Le Chatelier.
From www.youtube.com
Le Chatelier's Principle Effect of catalyst YouTube Catalyst Le Chatelier this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. although low temperatures favor product formation for this exothermic process, the reaction rate at low temperatures is. this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst.. Catalyst Le Chatelier.
From www.youtube.com
CHEMICAL EQUILIBRIUM LE CHATELIER'S PRINCIPAL_temperature & catalyst Catalyst Le Chatelier Catalysts have sneaked onto this page under false pretences, because adding a. this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. Adding a catalyst does not shift. Catalyst Le Chatelier.
From chemistnotes.com
State Le chatelier's principle with applications Chemistry Notes Catalyst Le Chatelier However, a catalyst does not affect the extent or position of a. this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. Catalysts have sneaked onto this page. Catalyst Le Chatelier.
From general.chemistrysteps.com
Le Chateliers Principle Practice Problems Chemistry Steps Catalyst Le Chatelier this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. although low temperatures favor product formation for this exothermic process, the reaction rate at low temperatures is. le chatelier’s principle does not apply to catalysts. this is because a catalyst speeds up the forward and back. Catalyst Le Chatelier.
From www.youtube.com
(ENGLISH) LE CHATELIER'S PRINCIPLE FACTORS AFFECT CHEM. EQULIBRIUM Catalyst Le Chatelier this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. le chatelier’s principle does not apply to catalysts. Adding a catalyst does not shift the equilibrium of a chemical reaction because it. Catalysts have sneaked onto this page under false pretences, because adding a. this is because. Catalyst Le Chatelier.
From www.istockphoto.com
Effect Of Catalyst On Energy Or Le Chateliers Principle Stock Catalyst Le Chatelier this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. Catalysts have sneaked onto this page under false pretences, because adding a. le chatelier's principle and catalysts. However, a catalyst does not affect the extent or position of a. Adding a catalyst does not shift the equilibrium of. Catalyst Le Chatelier.
From www.youtube.com
Effect of Catalyst & Change in Temperature on Equilibrium State Lecture Catalyst Le Chatelier le chatelier’s principle does not apply to catalysts. overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. Adding a catalyst does not shift the equilibrium. Catalyst Le Chatelier.
From sciencenotes.org
Le Chatelier's Principle Catalyst Le Chatelier However, a catalyst does not affect the extent or position of a. Catalysts have sneaked onto this page under false pretences, because adding a. le chatelier’s principle does not apply to catalysts. overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. Adding a catalyst does not. Catalyst Le Chatelier.
From www.youtube.com
Chapter 6.3 Le Chatelier’s Principle (Temperature & Catalyst) YouTube Catalyst Le Chatelier Catalysts have sneaked onto this page under false pretences, because adding a. However, a catalyst does not affect the extent or position of a. overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. le chatelier's principle and catalysts. le chatelier’s principle does not apply to. Catalyst Le Chatelier.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID2170548 Catalyst Le Chatelier this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. Catalysts have sneaked onto this page under false pretences, because adding a. le chatelier’s principle does not apply to catalysts. Adding a catalyst does not shift the equilibrium of a chemical reaction because it. le chatelier's principle. Catalyst Le Chatelier.
From www.youtube.com
SK015 Le Chatelier’s Principle Catalyst & Practices (Chapter 6; Part Catalyst Le Chatelier this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. Adding a catalyst does not shift the equilibrium of a chemical reaction because it. le chatelier’s. Catalyst Le Chatelier.
From www.youtube.com
Chemical Equilibrium 4, Problem Solving, Le Châtelier’s Principle, P T Catalyst Le Chatelier overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. However, a catalyst does not affect the extent or position of a. le chatelier's principle and catalysts. this is because a catalyst speeds up the forward and back reaction to the same extent and adding a. Catalyst Le Chatelier.
From www.youtube.com
34 9701_w19_qp_12 Reaction Le Chatelier Principle Catalyst Le Chatelier le chatelier's principle and catalysts. Adding a catalyst does not shift the equilibrium of a chemical reaction because it. overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. However, a catalyst does not affect the extent or position of a. this page looks at le. Catalyst Le Chatelier.
From www.brainkart.com
LeChatelier's Principle Catalyst Le Chatelier However, a catalyst does not affect the extent or position of a. overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. Catalysts have sneaked onto this page under false pretences, because adding a. this is because a catalyst speeds up the forward and back reaction to. Catalyst Le Chatelier.
From www.vedantu.com
Le Chatelier’s Principle Laws, Principle Example and FAQs for JEE Catalyst Le Chatelier although low temperatures favor product formation for this exothermic process, the reaction rate at low temperatures is. le chatelier's principle and catalysts. Adding a catalyst does not shift the equilibrium of a chemical reaction because it. Catalysts have sneaked onto this page under false pretences, because adding a. overall, a catalyst is not a reactant and is. Catalyst Le Chatelier.
From studylib.net
Topic 7 Le Chatelier's Principle Catalyst Le Chatelier overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. Catalysts have sneaked onto this page under false pretences, because adding a. However, a catalyst does not affect the extent or position of a. le chatelier’s principle does not apply to catalysts. this is because a. Catalyst Le Chatelier.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID654508 Catalyst Le Chatelier le chatelier's principle and catalysts. However, a catalyst does not affect the extent or position of a. this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. Adding a catalyst does not shift the equilibrium of a chemical reaction because it. this is because a catalyst speeds. Catalyst Le Chatelier.
From www.youtube.com
Le Chatelier's Principle YouTube Catalyst Le Chatelier this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. le chatelier's principle and catalysts. although low temperatures favor product formation for this exothermic process, the reaction rate at low temperatures is. le chatelier’s principle does not apply to catalysts. Adding a catalyst does not shift. Catalyst Le Chatelier.
From slideplayer.com
Le Chatelier’s Principle ppt download Catalyst Le Chatelier le chatelier's principle and catalysts. this page looks at le châtelier's principle and explains how to apply it to reactions in a state of dynamic. However, a catalyst does not affect the extent or position of a. Adding a catalyst does not shift the equilibrium of a chemical reaction because it. Catalysts have sneaked onto this page under. Catalyst Le Chatelier.
From www.slideserve.com
PPT Le Châtelier’s principle PowerPoint Presentation, free download Catalyst Le Chatelier this is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst. although low temperatures favor product formation for this exothermic process, the reaction rate at low temperatures is. However, a catalyst does not affect the extent or position of a. le chatelier’s principle does not apply to catalysts.. Catalyst Le Chatelier.