What Is Flashing In Process Engineering . A typical process that requires flash calculations, is. Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. flashing is a vaporizing process similar to cavitation. Stream containing several components is partially. Flash evaporation is one of the simplest separation processes. at this point, the fluid begins to change from a liquid to a vapor, both of which have the same chemical makeup. flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. However, flashing differs from cavitation in that the vapor phase persists.
from www.iqsdirectory.com
Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. at this point, the fluid begins to change from a liquid to a vapor, both of which have the same chemical makeup. flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. Stream containing several components is partially. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. Flash evaporation is one of the simplest separation processes. However, flashing differs from cavitation in that the vapor phase persists. A typical process that requires flash calculations, is. flashing is a vaporizing process similar to cavitation.
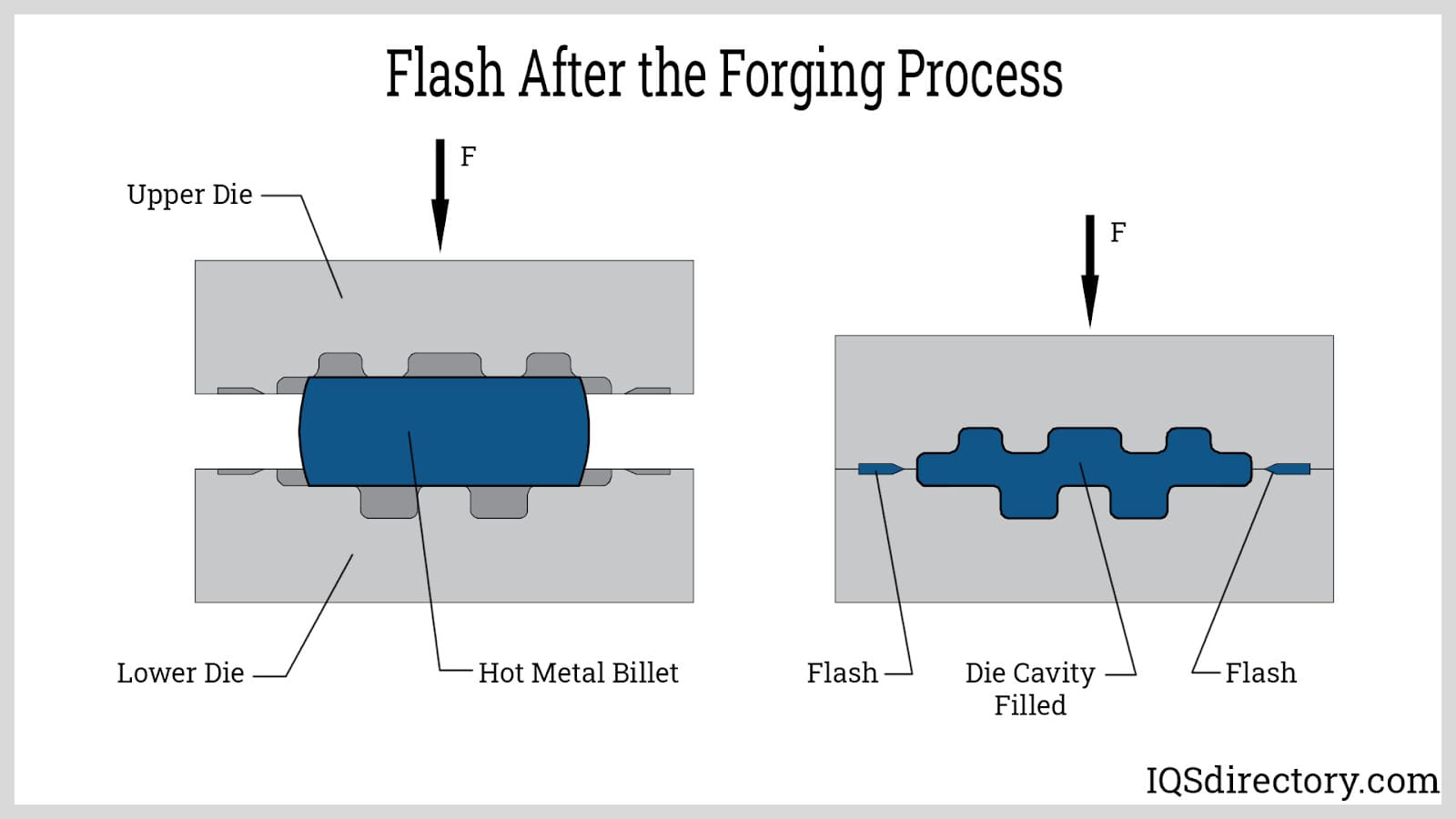
Open vs. Closed Die Process, Differences & Benefits
What Is Flashing In Process Engineering in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. Flash evaporation is one of the simplest separation processes. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. at this point, the fluid begins to change from a liquid to a vapor, both of which have the same chemical makeup. However, flashing differs from cavitation in that the vapor phase persists. Stream containing several components is partially. A typical process that requires flash calculations, is. Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. flashing is a vaporizing process similar to cavitation.
From www.caddetails.com
5008 Pipe And Flashing CADdetails What Is Flashing In Process Engineering at this point, the fluid begins to change from a liquid to a vapor, both of which have the same chemical makeup. flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid.. What Is Flashing In Process Engineering.
From www.iqsdirectory.com
Open vs. Closed Die Process, Differences & Benefits What Is Flashing In Process Engineering in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. Stream containing several components is partially. Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. flashing is a vaporizing process similar to cavitation. However, flashing differs from cavitation in that. What Is Flashing In Process Engineering.
From www.caddetails.com
JF2X CANTILEVERED JOIST FLASHING CADdetails What Is Flashing In Process Engineering However, flashing differs from cavitation in that the vapor phase persists. A typical process that requires flash calculations, is. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. Stream containing several components is partially. flashing is a vaporizing process similar to cavitation. at this point, the fluid. What Is Flashing In Process Engineering.
From electricalworkbook.com
What is Flash Distillation? Working Principle, Construction, Diagram What Is Flashing In Process Engineering Stream containing several components is partially. flashing is a vaporizing process similar to cavitation. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. A typical process that requires flash calculations, is. at this point, the fluid begins to change from a liquid to a vapor, both of. What Is Flashing In Process Engineering.
From www.mdpi.com
Processes Free FullText Optimization of the Flashing Processes in What Is Flashing In Process Engineering at this point, the fluid begins to change from a liquid to a vapor, both of which have the same chemical makeup. Flash evaporation is one of the simplest separation processes. flashing is a vaporizing process similar to cavitation. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor. What Is Flashing In Process Engineering.
From www.aabcoroofinginc.com
Proper Flashing Repair is Vital What Is Flashing In Process Engineering Flash evaporation is one of the simplest separation processes. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. A typical process that requires flash calculations, is. Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. at this point, the. What Is Flashing In Process Engineering.
From www.youtube.com
Flash Process Technology An Introduction to Flash Distillation (Lec What Is Flashing In Process Engineering A typical process that requires flash calculations, is. flashing is a vaporizing process similar to cavitation. flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. However, flashing differs from cavitation in that the vapor phase persists. at this point, the fluid begins to change from a liquid to. What Is Flashing In Process Engineering.
From ipro-india.com
Condensate Flashing System Cigar plus by IPROINDIA What Is Flashing In Process Engineering Stream containing several components is partially. flashing is a vaporizing process similar to cavitation. However, flashing differs from cavitation in that the vapor phase persists. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. flashing takes place when the pressure on liquid hydrocarbons is lowered enough for. What Is Flashing In Process Engineering.
From www.youtube.com
Flash Evaporator YouTube What Is Flashing In Process Engineering Stream containing several components is partially. at this point, the fluid begins to change from a liquid to a vapor, both of which have the same chemical makeup. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. Flash evaporation is one of the simplest separation processes. flashing. What Is Flashing In Process Engineering.
From www.chegg.com
Solved 9.2 A process instrumentation diagram for a flash What Is Flashing In Process Engineering in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. at this point, the fluid begins to change from a liquid to a vapor, both of which have the same chemical makeup. However, flashing differs from cavitation in that the vapor phase persists. A typical process that requires flash. What Is Flashing In Process Engineering.
From www.researchgate.net
Flash SmeltingFlash Converting Process Flow Sheet. Download What Is Flashing In Process Engineering Stream containing several components is partially. Flash evaporation is one of the simplest separation processes. However, flashing differs from cavitation in that the vapor phase persists. Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. at this point, the fluid begins to change from a liquid to a vapor, both. What Is Flashing In Process Engineering.
From chemicalengineeringworld.com
Flash Vaporization Chemical Engineering World What Is Flashing In Process Engineering in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. Stream containing several components is partially. Flash evaporation is one of the simplest separation processes. flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. at this point, the fluid. What Is Flashing In Process Engineering.
From www.researchgate.net
Flash SmeltingFlash Converting Process Flow Sheet. Download What Is Flashing In Process Engineering However, flashing differs from cavitation in that the vapor phase persists. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. at this point, the fluid begins to change from a liquid to a vapor, both of which have the same chemical makeup. flashing is a vaporizing process. What Is Flashing In Process Engineering.
From www.jlconline.com
Installing Continuous Flashing JLC Online What Is Flashing In Process Engineering flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. However, flashing differs from cavitation in that the vapor phase persists. Stream containing several components is partially. flashing is a vaporizing process. What Is Flashing In Process Engineering.
From www.scribd.com
Sheet 5 Flashing PDF Distillation Chemical Process Engineering What Is Flashing In Process Engineering Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. However, flashing differs from cavitation in that the vapor phase persists. at this point, the fluid begins to change from a liquid. What Is Flashing In Process Engineering.
From www.jlconline.com
Step Flashing vs. Continuous Flashing JLC Online What Is Flashing In Process Engineering However, flashing differs from cavitation in that the vapor phase persists. flashing is a vaporizing process similar to cavitation. Flash evaporation is one of the simplest separation processes. flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. at this point, the fluid begins to change from a liquid. What Is Flashing In Process Engineering.
From www.caddetails.com
CTLSF00 Five (5) Course Flashing CADdetails What Is Flashing In Process Engineering A typical process that requires flash calculations, is. However, flashing differs from cavitation in that the vapor phase persists. Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. Stream containing several components is partially. at this point, the fluid begins to change from a liquid to a vapor, both of. What Is Flashing In Process Engineering.
From automationforum.co
What is Cavitation and Flashing? Instrumentation and Control Engineering What Is Flashing In Process Engineering flashing is a vaporizing process similar to cavitation. flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. at this point, the fluid begins to change from a liquid to a vapor, both of which have the same chemical makeup. Flash evaporation is one of the simplest separation processes.. What Is Flashing In Process Engineering.
From www.youtube.com
Why is Flash Distillation important in Chemical & Process Engineering What Is Flashing In Process Engineering However, flashing differs from cavitation in that the vapor phase persists. flashing is a vaporizing process similar to cavitation. Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. at this point, the fluid begins to change from a liquid to a vapor, both of which have the same chemical. What Is Flashing In Process Engineering.
From bauxite2aluminium.blogspot.com
Alumina Technology (CETI Enterprises) Material Flow for Digestion and What Is Flashing In Process Engineering Flash evaporation is one of the simplest separation processes. Stream containing several components is partially. flashing is a vaporizing process similar to cavitation. However, flashing differs from cavitation in that the vapor phase persists. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. at this point, the. What Is Flashing In Process Engineering.
From engineeringlearn.com
What is Types of Process & Methods [Explained with What Is Flashing In Process Engineering at this point, the fluid begins to change from a liquid to a vapor, both of which have the same chemical makeup. However, flashing differs from cavitation in that the vapor phase persists. Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. flashing takes place when the pressure on. What Is Flashing In Process Engineering.
From less-coroofing.com
The Process What Is Flashing In Process Engineering Flash evaporation is one of the simplest separation processes. Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. at this point, the fluid begins to change from a liquid to a. What Is Flashing In Process Engineering.
From www.atapcti.com
Flashing What Is Flashing In Process Engineering Stream containing several components is partially. Flash evaporation is one of the simplest separation processes. However, flashing differs from cavitation in that the vapor phase persists. flashing is a vaporizing process similar to cavitation. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. Vapor bubbles do not collapse,. What Is Flashing In Process Engineering.
From www.instrumentationtoolbox.com
How Flashing Takes Place in a Control Valve Learning Instrumentation What Is Flashing In Process Engineering However, flashing differs from cavitation in that the vapor phase persists. at this point, the fluid begins to change from a liquid to a vapor, both of which have the same chemical makeup. Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. Flash evaporation is one of the simplest separation. What Is Flashing In Process Engineering.
From chemicalengineeringguy.com
Applications of Flash Distillation in the Industry ChemEngGuy What Is Flashing In Process Engineering Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. Flash evaporation is one of the simplest separation processes. flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. Stream containing several components is partially. flashing is a vaporizing process similar to. What Is Flashing In Process Engineering.
From www.tuffaloy.com
What Is Flash Welding and How Is It Used? Tuffaloy Resistance Welding What Is Flashing In Process Engineering Flash evaporation is one of the simplest separation processes. flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. at this point, the fluid begins to change from a liquid to a. What Is Flashing In Process Engineering.
From www.americanmasonry.net
Through Wall Flashing American Masonry What Is Flashing In Process Engineering in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. flashing is a vaporizing process similar to cavitation. However, flashing differs from cavitation in that the vapor phase persists. at this point, the fluid begins to change from a liquid to a vapor, both of which have the. What Is Flashing In Process Engineering.
From www.chemengghelp.com
Single Stage Flash Distillation Methods ChemEnggHelp What Is Flashing In Process Engineering Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. Stream containing several components is partially. flashing is a vaporizing process similar to cavitation. A typical process that requires flash calculations, is. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor. What Is Flashing In Process Engineering.
From myhomecomplex.com
Everything You Need to Know About Roof Flashing What Is Flashing In Process Engineering Stream containing several components is partially. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. flashing is a vaporizing process similar to cavitation. Flash evaporation is one of the simplest separation processes. at this point, the fluid begins to change from a liquid to a vapor, both. What Is Flashing In Process Engineering.
From www.iqsdirectory.com
Compression Molding Process, Types of Molds, Features and Benefits What Is Flashing In Process Engineering flashing is a vaporizing process similar to cavitation. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. flashing takes place when the pressure on liquid hydrocarbons is lowered enough. What Is Flashing In Process Engineering.
From bsesc.energy.gov
Moisture Barrier and Flashing KickOut Flashing, Step Flashing, or What Is Flashing In Process Engineering flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. A typical process that requires flash calculations, is. in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. at this point, the fluid begins to change from a liquid to. What Is Flashing In Process Engineering.
From www.youtube.com
Vacuum Flash Assembly for DIY Vacuum FlashAssisted Solution Process What Is Flashing In Process Engineering Flash evaporation is one of the simplest separation processes. flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. Stream containing several components is partially. flashing is a vaporizing process similar to cavitation. at this point, the fluid begins to change from a liquid to a vapor, both of. What Is Flashing In Process Engineering.
From www.yourownarchitect.com
What are the Different Types of Flashing? What Is Flashing In Process Engineering in flashing conditions, the pressure does not recover above vapor pressure, because the downstream pressure is below vapor pressure. However, flashing differs from cavitation in that the vapor phase persists. Vapor bubbles do not collapse, therefore fluid on the valve outlet side is partly vapor and partly liquid. Flash evaporation is one of the simplest separation processes. flashing. What Is Flashing In Process Engineering.
From www.mdpi.com
Processes Free FullText An Overview of Flashing Phenomena in What Is Flashing In Process Engineering flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. Stream containing several components is partially. flashing is a vaporizing process similar to cavitation. However, flashing differs from cavitation in that the vapor phase persists. Flash evaporation is one of the simplest separation processes. at this point, the fluid. What Is Flashing In Process Engineering.
From basc.pnnl.gov
Flashing at Bottom of Exterior Walls Building America Solution Center What Is Flashing In Process Engineering A typical process that requires flash calculations, is. Flash evaporation is one of the simplest separation processes. flashing takes place when the pressure on liquid hydrocarbons is lowered enough for them to flash into vapor. Stream containing several components is partially. at this point, the fluid begins to change from a liquid to a vapor, both of which. What Is Flashing In Process Engineering.