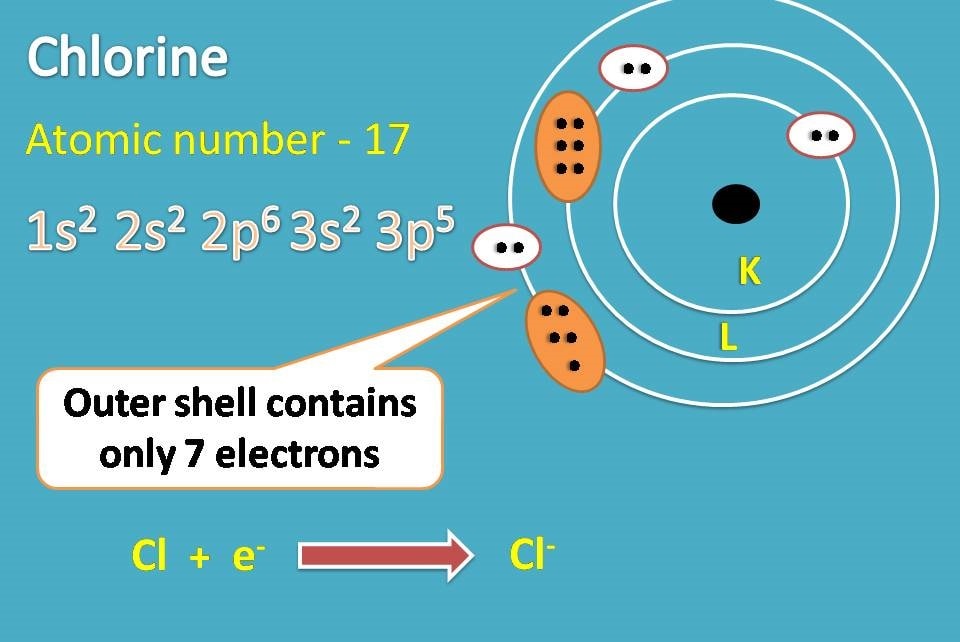
Chlorine Atom And Chlorine Ion . Tell students that when an atom gains or loses an electron, it becomes an ion. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Only one more electron is needed to achieve an octet in chlorine’s valence shell. In chemical bonding, a chlorine atom can share or transfer an electron, participating in covalent or ionic bonds. By removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Chloride ion is a chlorine atom that has gained. Atoms that gain extra electrons become. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. A chlorine atom starts with. Chlorine atom is an element with atomic number 17, meaning it has 17 protons in its nucleus and, when neutral, 17 electrons. Some elements, especially transition metals,. Chlorine makes ionic compounds in which the chloride ion always has a 1− charge. A chloride ion, with its extra electron, typically forms ionic. A neutral chlorine atom has seven electrons in its outermost shell. Since it has 1 more.
from enginelistchester.z5.web.core.windows.net
Atoms that gain extra electrons become. Some elements, especially transition metals,. Since it has 1 more. Chlorine makes ionic compounds in which the chloride ion always has a 1− charge. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. By removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. A chlorine atom starts with. In chemical bonding, a chlorine atom can share or transfer an electron, participating in covalent or ionic bonds. Naming anions is a little more complicated.
Chlorine Electron Dot Diagram
Chlorine Atom And Chlorine Ion Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. In chemical bonding, a chlorine atom can share or transfer an electron, participating in covalent or ionic bonds. Some elements, especially transition metals,. A chloride ion, with its extra electron, typically forms ionic. Atoms that gain extra electrons become. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. Chlorine atom is an element with atomic number 17, meaning it has 17 protons in its nucleus and, when neutral, 17 electrons. Since it has 1 more. A neutral chlorine atom has seven electrons in its outermost shell. Naming anions is a little more complicated. By removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. A chlorine atom starts with. Chlorine makes ionic compounds in which the chloride ion always has a 1− charge. Tell students that when an atom gains or loses an electron, it becomes an ion.
From periodictable.me
How To Find The Electron Configuration For Chlorine Dynamic Periodic Chlorine Atom And Chlorine Ion By removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. Chlorine atom is an element with atomic number 17, meaning it has 17 protons in its nucleus and, when. Chlorine Atom And Chlorine Ion.
From mavink.com
Bohr Model Of Chlorine Chlorine Atom And Chlorine Ion A chloride ion, with its extra electron, typically forms ionic. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Naming anions is a little more complicated. In chemical bonding, a chlorine atom can share or transfer an electron, participating in covalent or. Chlorine Atom And Chlorine Ion.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Atom And Chlorine Ion Only one more electron is needed to achieve an octet in chlorine’s valence shell. Atoms that gain extra electrons become. Chloride ion is a chlorine atom that has gained. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Some elements, especially transition metals,. A chloride ion, with its extra electron, typically forms ionic. In chemical. Chlorine Atom And Chlorine Ion.
From www.animalia-life.club
Chlorine Element Periodic Table Chlorine Atom And Chlorine Ion A chlorine atom starts with. A chloride ion, with its extra electron, typically forms ionic. Chlorine makes ionic compounds in which the chloride ion always has a 1− charge. Some elements, especially transition metals,. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. Chloride ion is a chlorine atom that has gained. Since it has 1. Chlorine Atom And Chlorine Ion.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Atom And Chlorine Ion Chlorine atom is an element with atomic number 17, meaning it has 17 protons in its nucleus and, when neutral, 17 electrons. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Some elements, especially transition metals,. By removing an electron from this atom we get a positively charged na + ion that has a net. Chlorine Atom And Chlorine Ion.
From utedzz.blogspot.com
Periodic Table Chlorine Atom Periodic Table Timeline Chlorine Atom And Chlorine Ion A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). A neutral chlorine atom has seven electrons in its outermost shell. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. Atoms that gain. Chlorine Atom And Chlorine Ion.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Atom And Chlorine Ion Atoms that gain extra electrons become. Naming anions is a little more complicated. Some elements, especially transition metals,. A chloride ion, with its extra electron, typically forms ionic. Chlorine atom is an element with atomic number 17, meaning it has 17 protons in its nucleus and, when neutral, 17 electrons. The ending of the element is typically dropped and replaced. Chlorine Atom And Chlorine Ion.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Atom And Chlorine Ion A neutral chlorine atom has seven electrons in its outermost shell. Some elements, especially transition metals,. In chemical bonding, a chlorine atom can share or transfer an electron, participating in covalent or ionic bonds. Tell students that when an atom gains or loses an electron, it becomes an ion. By removing an electron from this atom we get a positively. Chlorine Atom And Chlorine Ion.
From brainly.in
Draw the atomic structure of a chlorine ion Brainly.in Chlorine Atom And Chlorine Ion Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. A chlorine atom starts with. Only one more electron is needed to achieve an octet in chlorine’s valence shell. A chloride ion, with its extra electron, typically forms ionic. Since it has 1 more. Naming anions is a little more complicated. Some elements, especially transition metals,. A. Chlorine Atom And Chlorine Ion.
From mavink.com
Aufbau Diagram For Chlorine Chlorine Atom And Chlorine Ion A chlorine atom starts with. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Atoms that gain extra electrons become. Only one more electron is needed to achieve an octet in chlorine’s valence shell. In chemical bonding, a chlorine atom can share or transfer an electron, participating in covalent or ionic bonds. Since it has. Chlorine Atom And Chlorine Ion.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Chlorine Atom And Chlorine Ion In chemical bonding, a chlorine atom can share or transfer an electron, participating in covalent or ionic bonds. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Chlorine atom is an element with atomic number 17, meaning it has 17 protons in its nucleus and, when neutral, 17 electrons. The ending of the element is. Chlorine Atom And Chlorine Ion.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun Chlorine Atom And Chlorine Ion A chlorine atom starts with. A neutral chlorine atom has seven electrons in its outermost shell. Atoms that gain extra electrons become. Since it has 1 more. Chloride ion is a chlorine atom that has gained. Naming anions is a little more complicated. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the. Chlorine Atom And Chlorine Ion.
From www.alamy.com
Periodic table of elements chlorine hires stock photography and images Chlorine Atom And Chlorine Ion Tell students that when an atom gains or loses an electron, it becomes an ion. A chlorine atom starts with. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. By removing an electron from this atom we get a positively charged na + ion that has a net charge of. Chlorine Atom And Chlorine Ion.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Chlorine Atom And Chlorine Ion A chlorine atom starts with. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Tell students that when an atom gains or loses an electron, it becomes an ion. In chemical bonding, a chlorine atom can share or transfer an electron, participating in covalent or ionic bonds. Since it has 1 more. Chlorine makes ionic. Chlorine Atom And Chlorine Ion.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure Chlorine Atom And Chlorine Ion By removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Some elements, especially transition metals,. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. Naming. Chlorine Atom And Chlorine Ion.
From sciencenotes.org
Chlorine Facts Chlorine Atom And Chlorine Ion Some elements, especially transition metals,. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. By removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. A. Chlorine Atom And Chlorine Ion.
From socratic.org
Chlorine combined with two negative atom or 1 positive and other Chlorine Atom And Chlorine Ion Tell students that when an atom gains or loses an electron, it becomes an ion. Some elements, especially transition metals,. A neutral chlorine atom has seven electrons in its outermost shell. Atoms that gain extra electrons become. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Chlorine makes ionic compounds in which the chloride ion. Chlorine Atom And Chlorine Ion.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Atom And Chlorine Ion Since it has 1 more. Atoms that gain extra electrons become. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. In chemical bonding, a chlorine atom can share or transfer an electron, participating in covalent or ionic bonds. By removing an electron from this atom we get a positively charged. Chlorine Atom And Chlorine Ion.
From delorescgough.blob.core.windows.net
Chlorine Electron Charge at delorescgough blog Chlorine Atom And Chlorine Ion Since it has 1 more. A neutral chlorine atom has seven electrons in its outermost shell. In chemical bonding, a chlorine atom can share or transfer an electron, participating in covalent or ionic bonds. A chloride ion, with its extra electron, typically forms ionic. Chlorine atom is an element with atomic number 17, meaning it has 17 protons in its. Chlorine Atom And Chlorine Ion.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Chlorine Atom And Chlorine Ion Naming anions is a little more complicated. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. Chlorine makes ionic compounds in which the chloride ion always has a 1− charge. Chloride ion is a chlorine atom that has gained. By removing an. Chlorine Atom And Chlorine Ion.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Atom And Chlorine Ion Chlorine makes ionic compounds in which the chloride ion always has a 1− charge. A neutral chlorine atom has seven electrons in its outermost shell. Since it has 1 more. Tell students that when an atom gains or loses an electron, it becomes an ion. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Naming. Chlorine Atom And Chlorine Ion.
From www.britannica.com
Chlorine Uses, Properties, & Facts Britannica Chlorine Atom And Chlorine Ion Tell students that when an atom gains or loses an electron, it becomes an ion. A chloride ion, with its extra electron, typically forms ionic. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. Naming anions is a little more complicated. Atoms that gain extra electrons become. In chemical bonding,. Chlorine Atom And Chlorine Ion.
From pediaa.com
Difference Between Chlorine and Chloride Definition, Properties Chlorine Atom And Chlorine Ion Atoms that gain extra electrons become. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. A chloride ion, with its extra electron, typically forms ionic. By removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Some elements, especially transition metals,. Only one more electron. Chlorine Atom And Chlorine Ion.
From www.dreamstime.com
An Atom of Chlorine Diagram Stock Vector Illustration of structure Chlorine Atom And Chlorine Ion A chlorine atom starts with. Atoms that gain extra electrons become. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. Chlorine makes ionic compounds in which the chloride ion always has a 1− charge. A neutral chlorine atom has seven electrons in its outermost shell. Chloride ion is a chlorine. Chlorine Atom And Chlorine Ion.
From www.snexplores.org
Explainer Ions and radicals in our world Chlorine Atom And Chlorine Ion Chlorine atom is an element with atomic number 17, meaning it has 17 protons in its nucleus and, when neutral, 17 electrons. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. Tell students that when an atom gains or loses an electron, it becomes an ion. Chloride ion is a chlorine atom that has gained. In. Chlorine Atom And Chlorine Ion.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Chlorine Atom And Chlorine Ion Some elements, especially transition metals,. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. Chlorine atom is an element with atomic number 17, meaning it has 17 protons in its nucleus and, when neutral, 17 electrons. Naming anions is a little more complicated. In chemical bonding, a chlorine atom can share or transfer an electron, participating. Chlorine Atom And Chlorine Ion.
From oerpub.github.io
The top panel of this figure shows the orbit model of a sodium atom and Chlorine Atom And Chlorine Ion A chlorine atom starts with. By removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Some elements, especially transition metals,. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Chloride ion is a chlorine atom that has gained. Sodium loses an electron, leaving. Chlorine Atom And Chlorine Ion.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Chlorine Atom And Chlorine Ion A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. Naming anions is a little more complicated. A chlorine atom starts with. Chlorine atom is an element with atomic number 17, meaning it has 17 protons in. Chlorine Atom And Chlorine Ion.
From enginelistchester.z5.web.core.windows.net
Chlorine Electron Dot Diagram Chlorine Atom And Chlorine Ion Some elements, especially transition metals,. A neutral chlorine atom has seven electrons in its outermost shell. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. In chemical bonding, a chlorine atom can share or transfer an electron, participating in covalent or ionic bonds. Atoms that gain extra electrons become. A. Chlorine Atom And Chlorine Ion.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Chlorine Atom And Chlorine Ion Since it has 1 more. Chloride ion is a chlorine atom that has gained. Atoms that gain extra electrons become. A neutral chlorine atom has seven electrons in its outermost shell. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. Chlorine makes ionic compounds in which the chloride ion always. Chlorine Atom And Chlorine Ion.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chlorine Atom And Chlorine Ion Chloride ion is a chlorine atom that has gained. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). A neutral chlorine atom has seven electrons in its outermost shell. A chlorine atom starts with. Sodium loses. Chlorine Atom And Chlorine Ion.
From www.doubtnut.com
(a) Give the schematic atomic structures of chlorine atom and chloride Chlorine Atom And Chlorine Ion Some elements, especially transition metals,. In chemical bonding, a chlorine atom can share or transfer an electron, participating in covalent or ionic bonds. A chlorine atom starts with. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Atoms that gain extra electrons become. Naming anions is a little more complicated. Sodium loses an electron, leaving. Chlorine Atom And Chlorine Ion.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Atom And Chlorine Ion Some elements, especially transition metals,. A chlorine atom starts with. A chloride ion, with its extra electron, typically forms ionic. Naming anions is a little more complicated. Chloride ion is a chlorine atom that has gained. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Tell students that when an atom gains or loses an. Chlorine Atom And Chlorine Ion.
From circuitdataboattrains.z14.web.core.windows.net
Chlorine Atom Diagram Chlorine Atom And Chlorine Ion Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. By removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Some elements, especially transition metals,. Chloride ion is a chlorine atom that has gained. In chemical bonding, a chlorine atom can share or transfer an. Chlorine Atom And Chlorine Ion.
From loeylrncp.blob.core.windows.net
Chlorine Electrons And Protons at Edward Thompson blog Chlorine Atom And Chlorine Ion Atoms that gain extra electrons become. By removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. A neutral chlorine atom has seven electrons in its outermost shell. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. Since it has 1 more. Chlorine makes ionic. Chlorine Atom And Chlorine Ion.