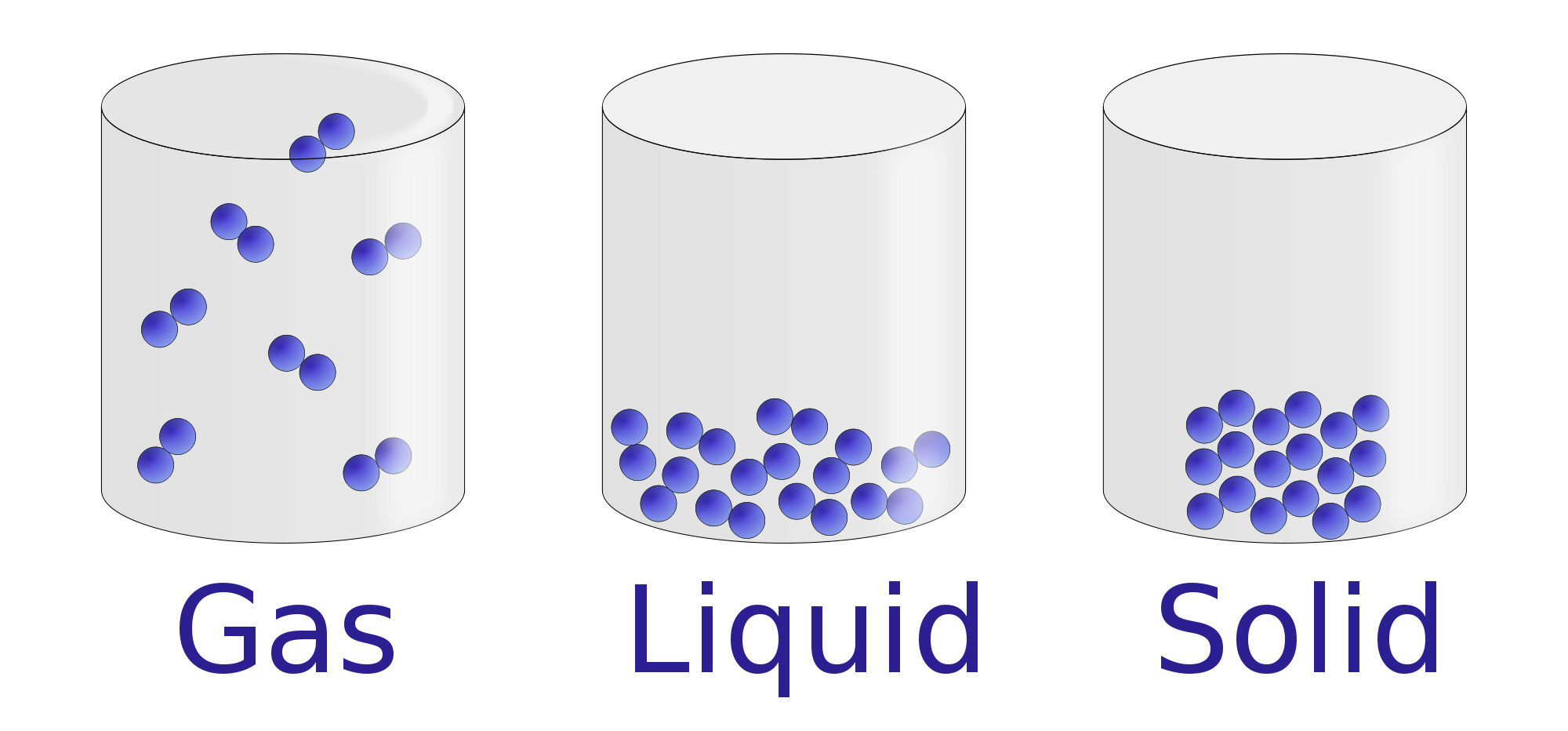
Solid Liquid Gas Kinetic Energy . If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. In general covalent bonds determine: The theory applies specifically to a model of a gas called an ideal gas. The theory helps explain observable properties and behaviors of solids, liquids, and gases. Changes of gaseous state to more condensed phases: In terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are somewhere in between. Ionic compounds never exist as gases since very high temperature is. All particles have energy, and the energy varies depending on the temperature the sample of matter is in, which determines if the substance is a solid, liquid, or gas. (all compared at the same. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The amount of energy required to sublime 1 mol of a pure solid is. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Liquids have more kinetic energy than solids. However, the theory is most easily understood as it applies to gases.
from www.visionlearning.com
Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The theory applies specifically to a model of a gas called an ideal gas. (all compared at the same. The amount of energy required to sublime 1 mol of a pure solid is. Liquids have more kinetic energy than solids. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. In terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are somewhere in between. In general covalent bonds determine: However, the theory is most easily understood as it applies to gases.
Properties of Liquids Chemistry Visionlearning
Solid Liquid Gas Kinetic Energy However, the theory is most easily understood as it applies to gases. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. In terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are somewhere in between. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. In general covalent bonds determine: The theory applies specifically to a model of a gas called an ideal gas. Liquids have more kinetic energy than solids. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. All particles have energy, and the energy varies depending on the temperature the sample of matter is in, which determines if the substance is a solid, liquid, or gas. Changes of gaseous state to more condensed phases: (all compared at the same. However, the theory is most easily understood as it applies to gases. The amount of energy required to sublime 1 mol of a pure solid is. The theory helps explain observable properties and behaviors of solids, liquids, and gases. Ionic compounds never exist as gases since very high temperature is.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Solid Liquid Gas Kinetic Energy The theory applies specifically to a model of a gas called an ideal gas. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. However, the theory is most easily understood as it applies. Solid Liquid Gas Kinetic Energy.
From sciencenotes.org
Examples of Gases What Is a Gas? Solid Liquid Gas Kinetic Energy In terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are somewhere in between. The amount of energy required to sublime 1 mol of a pure solid is. All particles have energy, and the energy varies depending on the temperature the sample of matter is in, which determines if the. Solid Liquid Gas Kinetic Energy.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Solid Liquid Gas Kinetic Energy If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. The amount of energy required to sublime 1 mol of a pure solid is. Changes of gaseous state to more condensed phases: All particles have energy, and the energy varies depending on the temperature the sample of matter is in, which. Solid Liquid Gas Kinetic Energy.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Solid Liquid Gas Kinetic Energy Changes of gaseous state to more condensed phases: In terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are somewhere in between. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. If you add heat energy to a liquid, the particles. Solid Liquid Gas Kinetic Energy.
From brainly.in
How does the energy of solids, liquids, and gases compare Solid Liquid Gas Kinetic Energy If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. All particles have energy, and the energy varies depending on the temperature the sample of matter is in, which determines if the substance is a solid, liquid, or gas. In general covalent bonds determine: Heat, cool and compress atoms and molecules. Solid Liquid Gas Kinetic Energy.
From www.youtube.com
States of Matter Solid Liquid Gas States of Matter drawing Different Solid Liquid Gas Kinetic Energy (all compared at the same. In terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are somewhere in between. However, the theory is most easily understood as it applies to gases. The amount of energy required to sublime 1 mol of a pure solid is. Liquids have more kinetic energy. Solid Liquid Gas Kinetic Energy.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Solid Liquid Gas Kinetic Energy The theory helps explain observable properties and behaviors of solids, liquids, and gases. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. In terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are somewhere in between. The amount of energy required to. Solid Liquid Gas Kinetic Energy.
From www.slideshare.net
Particle Theory (Slg Introduction) Solid Liquid Gas Kinetic Energy If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. However, the theory is most easily understood as it applies to gases. Liquids have more kinetic energy than solids. (all compared at the same.. Solid Liquid Gas Kinetic Energy.
From www.snexplores.org
Explainer What are the different states of matter? Solid Liquid Gas Kinetic Energy The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Liquids have more kinetic energy than solids. The amount of energy required to sublime 1 mol of a pure solid is. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. Changes of gaseous. Solid Liquid Gas Kinetic Energy.
From sciencenotes.org
States of Matter Solid Liquid Gas Kinetic Energy The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Liquids have more kinetic energy than solids. The theory applies specifically to a model of a gas called an ideal gas. All particles have energy, and the energy varies depending on the temperature the sample of matter is in, which determines if the. Solid Liquid Gas Kinetic Energy.
From guidelistbaquantising.z13.web.core.windows.net
Venn Diagram For Solids Liquids And Gases Solid Liquid Gas Kinetic Energy Changes of gaseous state to more condensed phases: The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Liquids have more kinetic energy than solids. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The theory helps explain observable properties and behaviors of solids,. Solid Liquid Gas Kinetic Energy.
From www.slideserve.com
PPT The Theory of Matter explains the properties of solids Solid Liquid Gas Kinetic Energy In general covalent bonds determine: In terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are somewhere in between. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. The direct conversion of a solid to a gas, without an intervening. Solid Liquid Gas Kinetic Energy.
From diagramlibrarywany.z19.web.core.windows.net
Solid Liquid And Gas Particle Diagram Solid Liquid Gas Kinetic Energy The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Ionic compounds never exist as gases since very high temperature is. The amount of energy required to sublime 1 mol of a pure solid is. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases.. Solid Liquid Gas Kinetic Energy.
From slideplayer.com
States of Matter & Energy ppt download Solid Liquid Gas Kinetic Energy Changes of gaseous state to more condensed phases: If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. All particles have energy, and the energy varies depending on the temperature the sample of matter is in, which determines if the substance is a solid, liquid, or gas. Liquids have more kinetic. Solid Liquid Gas Kinetic Energy.
From www.slideserve.com
PPT Solids, Liquids, and Gases PowerPoint Presentation, free download Solid Liquid Gas Kinetic Energy The amount of energy required to sublime 1 mol of a pure solid is. (all compared at the same. However, the theory is most easily understood as it applies to gases. Changes of gaseous state to more condensed phases: In general covalent bonds determine: In terms of relative energy, gas particles have the most energy, solid particles have the least. Solid Liquid Gas Kinetic Energy.
From gideonkruwcantrell.blogspot.com
Describe Solids Liquids and Gases Using the Theory Solid Liquid Gas Kinetic Energy If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. Changes of gaseous state to more condensed phases: (all compared at the same. The theory helps explain observable properties and behaviors of solids, liquids, and gases. The amount of energy required to sublime 1 mol of a pure solid is. In. Solid Liquid Gas Kinetic Energy.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Solid Liquid Gas Kinetic Energy In general covalent bonds determine: Ionic compounds never exist as gases since very high temperature is. However, the theory is most easily understood as it applies to gases. The theory helps explain observable properties and behaviors of solids, liquids, and gases. (all compared at the same. If you add heat energy to a liquid, the particles will move faster around. Solid Liquid Gas Kinetic Energy.
From www.slideserve.com
PPT Matter Theory Solid Liquid Gas Plasma PowerPoint Solid Liquid Gas Kinetic Energy Liquids have more kinetic energy than solids. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. In general covalent bonds determine: Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. However, the theory is most easily understood as it applies to. Solid Liquid Gas Kinetic Energy.
From www.pinterest.com
States of matter can be more than just your average solids, liquids and Solid Liquid Gas Kinetic Energy Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. However, the theory is most easily understood as it applies to gases. Changes of gaseous state to more condensed phases: If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. Ionic compounds never. Solid Liquid Gas Kinetic Energy.
From gcsephysicsninja.com
3. Energy of solids, liquids and gases Solid Liquid Gas Kinetic Energy If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. In terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are somewhere in between.. Solid Liquid Gas Kinetic Energy.
From msreinders.weebly.com
States of Matter Shaw Middle School Science Solid Liquid Gas Kinetic Energy (all compared at the same. The theory applies specifically to a model of a gas called an ideal gas. All particles have energy, and the energy varies depending on the temperature the sample of matter is in, which determines if the substance is a solid, liquid, or gas. If you add heat energy to a liquid, the particles will move. Solid Liquid Gas Kinetic Energy.
From www.shutterstock.com
Theory Matter Infographic Diagram Showing Stock Illustration Solid Liquid Gas Kinetic Energy In terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are somewhere in between. The amount of energy required to sublime 1 mol of a pure solid is. However, the theory is most easily understood as it applies to gases. If you add heat energy to a liquid, the particles. Solid Liquid Gas Kinetic Energy.
From www.slideserve.com
PPT Density PowerPoint Presentation, free download ID5878733 Solid Liquid Gas Kinetic Energy Liquids have more kinetic energy than solids. The theory applies specifically to a model of a gas called an ideal gas. Changes of gaseous state to more condensed phases: If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. The theory helps explain observable properties and behaviors of solids, liquids, and. Solid Liquid Gas Kinetic Energy.
From www.slideserve.com
PPT Chapter 16 Solids, Liquids, and Gases Section 1 Theory Solid Liquid Gas Kinetic Energy The amount of energy required to sublime 1 mol of a pure solid is. In general covalent bonds determine: However, the theory is most easily understood as it applies to gases. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. (all compared at the same. In terms of relative energy, gas particles. Solid Liquid Gas Kinetic Energy.
From www.docbrown.info
GASES LIQUIDS SOLIDS States of Matter, particle theory models Solid Liquid Gas Kinetic Energy The theory helps explain observable properties and behaviors of solids, liquids, and gases. Liquids have more kinetic energy than solids. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. All particles have energy, and the energy varies depending on the temperature the sample of matter is in, which determines if. Solid Liquid Gas Kinetic Energy.
From www.youtube.com
theory of Solids, Liquids & Gases IGCSE Chemistry Learn Solid Liquid Gas Kinetic Energy In general covalent bonds determine: (all compared at the same. However, the theory is most easily understood as it applies to gases. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The theory helps explain observable properties and behaviors of solids, liquids, and gases. Ionic compounds never exist as gases since. Solid Liquid Gas Kinetic Energy.
From slideplayer.com
Solid Gas Liquid INTERNAL ENERGY, U ppt download Solid Liquid Gas Kinetic Energy If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. The theory helps explain observable properties and behaviors of solids, liquids, and gases. Changes of gaseous state to more condensed phases: Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Ionic compounds. Solid Liquid Gas Kinetic Energy.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Solid Liquid Gas Kinetic Energy Ionic compounds never exist as gases since very high temperature is. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Liquids have more kinetic energy than solids. (all compared at the same. All particles have energy, and the energy varies depending on the temperature the sample of matter is in, which. Solid Liquid Gas Kinetic Energy.
From brainly.com
How does the energy of solids, liquids, and gases compare? OA Solid Liquid Gas Kinetic Energy If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. However, the theory is most easily understood as it applies to gases. Liquids have more kinetic energy than solids. (all compared at the same.. Solid Liquid Gas Kinetic Energy.
From gcsephysicsninja.com
9. Relative expansion of solids, liquids and gases Solid Liquid Gas Kinetic Energy Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The theory applies specifically to a model of a gas called an ideal gas. However, the theory is most easily understood as it applies to gases. Liquids have more kinetic energy than solids. If you add heat energy to a liquid, the. Solid Liquid Gas Kinetic Energy.
From joilgqxpm.blob.core.windows.net
Solids Liquids And Gases Ks2 at Eric Lee blog Solid Liquid Gas Kinetic Energy The theory helps explain observable properties and behaviors of solids, liquids, and gases. However, the theory is most easily understood as it applies to gases. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic. The amount of energy required to sublime 1 mol of a pure solid is. Ionic compounds. Solid Liquid Gas Kinetic Energy.
From www.slideserve.com
PPT Molecular Theory States of Matter Phase Changes Solid Liquid Gas Kinetic Energy In general covalent bonds determine: The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. In terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are somewhere in between. The amount of energy required to sublime 1 mol of a pure solid is.. Solid Liquid Gas Kinetic Energy.
From www.tes.com
theory of solids, liquids and gases Teaching Resources Solid Liquid Gas Kinetic Energy In terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are somewhere in between. In general covalent bonds determine: Liquids have more kinetic energy than solids. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Ionic compounds never exist as gases since. Solid Liquid Gas Kinetic Energy.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solid Liquid Gas Kinetic Energy However, the theory is most easily understood as it applies to gases. The theory applies specifically to a model of a gas called an ideal gas. The amount of energy required to sublime 1 mol of a pure solid is. In general covalent bonds determine: The theory helps explain observable properties and behaviors of solids, liquids, and gases. The direct. Solid Liquid Gas Kinetic Energy.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID5445725 Solid Liquid Gas Kinetic Energy The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Changes of gaseous state to more condensed phases: The theory applies specifically to a model of a gas called an ideal gas. Liquids have more. Solid Liquid Gas Kinetic Energy.