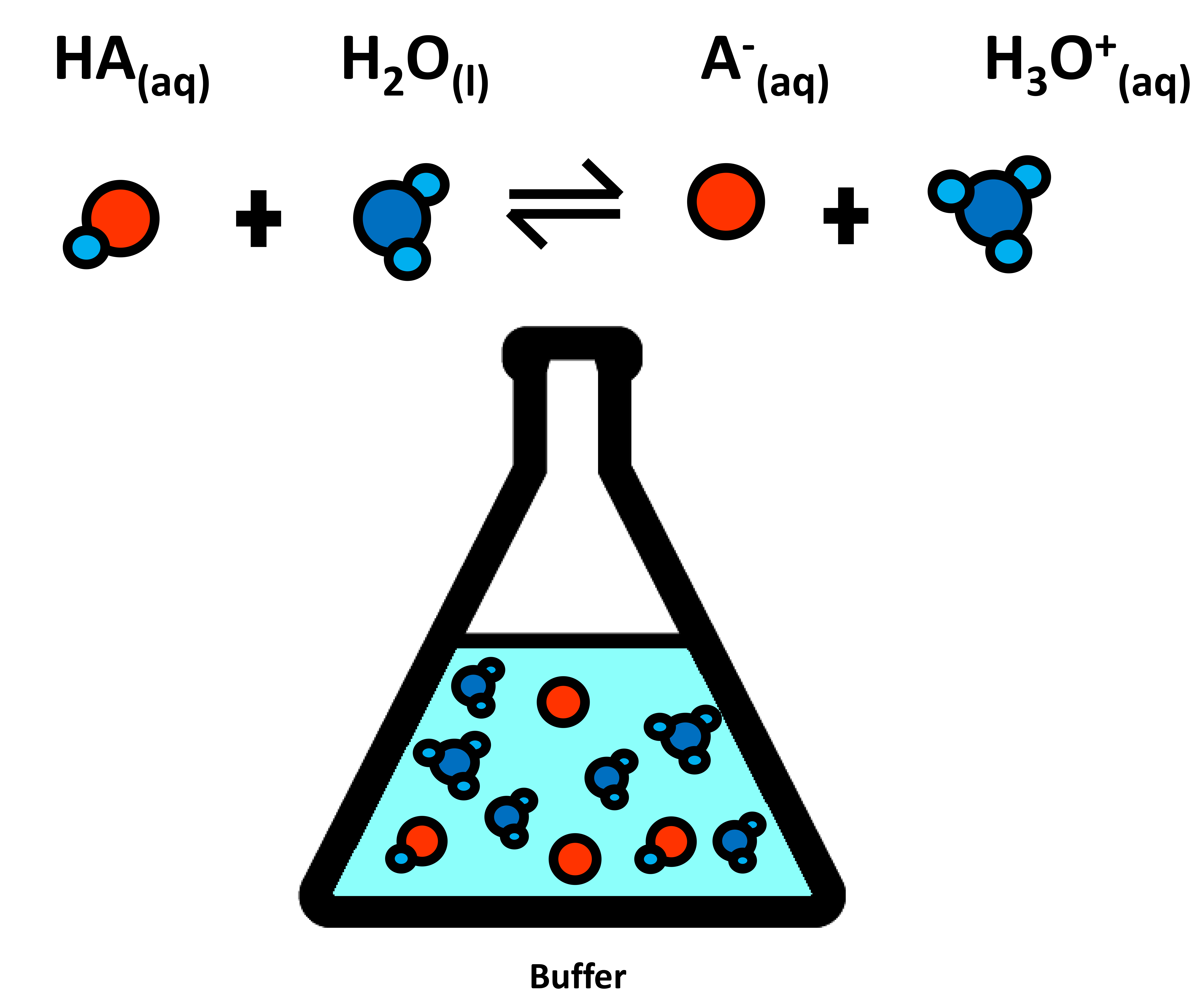
What Is Buffer In Botany . A buffer is a solution that resists a significant change in ph upon addition. Buffers • biological systems use buffers to maintain ph. What is buffer in chemistry? A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer is a solution that maintains the stability of a system’s ph level when adding. Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is.
from dxobuticl.blob.core.windows.net
What is buffer in chemistry? In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa. A buffer is a solution that resists a significant change in ph upon addition. Buffers • biological systems use buffers to maintain ph. A buffer is a solution that maintains the stability of a system’s ph level when adding. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation.
Commonly Used Buffers In The Laboratory at Savannah Osgood blog
What Is Buffer In Botany A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer is a solution that resists a significant change in ph upon addition. Buffers • biological systems use buffers to maintain ph. What is buffer in chemistry? In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa. A buffer is a solution that maintains the stability of a system’s ph level when adding.
From ctrivergateway.org
Riparian Buffers and Landscape Plantings Connecticut River Gateway What Is Buffer In Botany Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Buffers • biological systems use buffers to maintain ph. In other words, a buffer solution. What Is Buffer In Botany.
From www.tffn.net
What is a Buffer in Science? Exploring the Role of Buffers in Chemistry What Is Buffer In Botany What is buffer in chemistry? A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice. What Is Buffer In Botany.
From modernbio.com
Electrophoresis Buffer Modern Biology, Inc. What Is Buffer In Botany In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa. Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. A buffer is a solution that maintains. What Is Buffer In Botany.
From www.brainkart.com
Buffers What Is Buffer In Botany Buffers • biological systems use buffers to maintain ph. A buffer is a solution that maintains the stability of a system’s ph level when adding. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at. What Is Buffer In Botany.
From www.youtube.com
BufferAcidic & basic buffer , Reactions and factors affecting pH of What Is Buffer In Botany In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa. A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer is a solution that resists a significant. What Is Buffer In Botany.
From www.peoi.org
Chapter 12 Section G Buffers What Is Buffer In Botany A buffer is a solution that resists a significant change in ph upon addition. What is buffer in chemistry? Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. A solution whose ph is not altered to any great extent by the addition of small quantities of either an. What Is Buffer In Botany.
From loenamchg.blob.core.windows.net
What Is A Buffer For Ph at Wesley Fuqua blog What Is Buffer In Botany A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa. What is buffer in. What Is Buffer In Botany.
From joifezsdx.blob.core.windows.net
Biological Buffers Ppt at Erin Dodds blog What Is Buffer In Botany A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. What is buffer in chemistry? Buffers • biological systems use buffers to maintain ph. Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. A. What Is Buffer In Botany.
From wetlandinfo-test.des.qld.gov.au
Vegetated buffers and swales — Planning and design (Department of What Is Buffer In Botany A buffer is a solution that resists a significant change in ph upon addition. In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa. Buffers • biological systems use buffers to maintain ph. A buffer is a solution. What Is Buffer In Botany.
From dxouambuf.blob.core.windows.net
How To Use A Buffer at Albert Hill blog What Is Buffer In Botany In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa. Buffers • biological systems use buffers to maintain ph. A buffer is a solution that resists a significant change in ph upon addition. A buffer is a solution. What Is Buffer In Botany.
From dxobuticl.blob.core.windows.net
Commonly Used Buffers In The Laboratory at Savannah Osgood blog What Is Buffer In Botany A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer solution resists a change in ph from the addition of a small amount of acid or base. In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is. What Is Buffer In Botany.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses What Is Buffer In Botany A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. A solution whose ph is not altered to any great extent by the addition of small quantities of either an. What Is Buffer In Botany.
From www.youtube.com
Types of buffers carbonic acid buffer phosphate buffer protein amino What Is Buffer In Botany Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. A buffer solution resists a change in ph from the addition of a small amount of acid or base. In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution. What Is Buffer In Botany.
From www.musimmas.com
How Do Riparian Buffers Protect the Environment? Musim Mas What Is Buffer In Botany Buffers • biological systems use buffers to maintain ph. What is buffer in chemistry? A buffer is a solution that resists a significant change in ph upon addition. In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa.. What Is Buffer In Botany.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID7055849 What Is Buffer In Botany A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer is a solution that maintains the stability of a system’s ph level when adding. In. What Is Buffer In Botany.
From exyeghjzx.blob.core.windows.net
What Are The Types Of Buffers at Virginia Saunders blog What Is Buffer In Botany Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. A buffer is a solution that resists a significant change in ph upon addition. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. What. What Is Buffer In Botany.
From slideplayer.com
S.Y.BSc SEMESTER III BOTANY PAPER II ppt download What Is Buffer In Botany In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa. Buffers • biological systems use buffers to maintain ph. A buffer is a solution that maintains the stability of a system’s ph level when adding. What is buffer. What Is Buffer In Botany.
From slideplayer.com
S.Y.BSc SEMESTER III BOTANY PAPER II ppt download What Is Buffer In Botany Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. A buffer solution resists a change in ph from the addition of a small amount of acid or base. In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution. What Is Buffer In Botany.
From loeukppln.blob.core.windows.net
Buffers Ap Biology at Venessa Baird blog What Is Buffer In Botany In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa. Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. A buffer is a solution that resists. What Is Buffer In Botany.
From www.worksheetsplanet.com
What is Botany Definition of Botany What Is Buffer In Botany A buffer is a solution that resists a significant change in ph upon addition. A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer is a solution that maintains the stability of a system’s ph level when adding. In other words, a buffer solution (also known as a ph. What Is Buffer In Botany.
From woodlandinfo.org
Riparian Buffers Agroforestry for Any Property UWMadison Extension What Is Buffer In Botany A buffer is a solution that resists a significant change in ph upon addition. A buffer is a solution that maintains the stability of a system’s ph level when adding. A buffer solution resists a change in ph from the addition of a small amount of acid or base. A solution whose ph is not altered to any great extent. What Is Buffer In Botany.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID2813415 What Is Buffer In Botany A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer is a solution that resists a significant change in ph upon addition. Buffers • biological systems use buffers to maintain ph. A buffer solution resists a change in ph from the addition of a. What Is Buffer In Botany.
From www.youtube.com
ph and buffer Biomolecules & cell biology CUETPG Bsc botany1st Sem What Is Buffer In Botany A buffer solution resists a change in ph from the addition of a small amount of acid or base. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer is a solution that resists a significant change in ph upon addition. Buffers • biological. What Is Buffer In Botany.
From www.theherringpondswatershed.org
Why do I need a Vegetated Buffer? Herring Ponds Watershed Association What Is Buffer In Botany A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. What is buffer in chemistry? Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. A buffer solution resists a change in ph from the. What Is Buffer In Botany.
From cedggslh.blob.core.windows.net
What Is A Buffer How Does It Form at Lloyd Limon blog What Is Buffer In Botany What is buffer in chemistry? A buffer is a solution that resists a significant change in ph upon addition. A buffer is a solution that maintains the stability of a system’s ph level when adding. Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. In other words, a. What Is Buffer In Botany.
From www.troutheadwaters.com
What is a "Riparian Buffer Zone" and Why is it Important? What Is Buffer In Botany A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. Buffers • biological systems use buffers to maintain ph. A buffer is a solution that maintains the stability of a. What Is Buffer In Botany.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Is Buffer In Botany What is buffer in chemistry? Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. A buffer is a solution that maintains the stability of a system’s ph level when adding. A buffer is a solution that resists a significant change in ph upon addition. A buffer solution resists. What Is Buffer In Botany.
From www.youtube.com
Botany Class 3 pH, Buffer & Water 23.03.2024 YouTube What Is Buffer In Botany A buffer is a solution that maintains the stability of a system’s ph level when adding. What is buffer in chemistry? A buffer solution resists a change in ph from the addition of a small amount of acid or base. In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution. What Is Buffer In Botany.
From www.slideshare.net
Buffer system What Is Buffer In Botany In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa. A buffer is a solution that maintains the stability of a system’s ph level when adding. Buffers are used in microscopy to reduce the production of artefacts by. What Is Buffer In Botany.
From exyeghjzx.blob.core.windows.net
What Are The Types Of Buffers at Virginia Saunders blog What Is Buffer In Botany In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa. A buffer is a solution that maintains the stability of a system’s ph level when adding. Buffers are used in microscopy to reduce the production of artefacts by. What Is Buffer In Botany.
From slideplayer.com
S.Y.BSc SEMESTER III BOTANY PAPER II ppt download What Is Buffer In Botany A buffer is a solution that maintains the stability of a system’s ph level when adding. What is buffer in chemistry? In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa. A solution whose ph is not altered. What Is Buffer In Botany.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint What Is Buffer In Botany Buffers • biological systems use buffers to maintain ph. A buffer is a solution that resists a significant change in ph upon addition. Buffers are used in microscopy to reduce the production of artefacts by maintaining the specimen at its original ph during fixation. A solution whose ph is not altered to any great extent by the addition of small. What Is Buffer In Botany.
From slideplayer.com
S.Y.BSc SEMESTER III BOTANY PAPER II ppt download What Is Buffer In Botany A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers • biological systems use buffers to maintain ph. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Buffers are used in microscopy to reduce the. What Is Buffer In Botany.
From www.youtube.com
lecture of buffers botany D chapter 1 by prof riaz ul haq ramay What Is Buffer In Botany In other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa. Buffers • biological systems use buffers to maintain ph. A buffer is a solution that resists a significant change in ph upon addition. A solution whose ph is. What Is Buffer In Botany.
From slideplayer.com
S.Y.BSc SEMESTER III BOTANY PAPER II ppt download What Is Buffer In Botany Buffers • biological systems use buffers to maintain ph. A buffer is a solution that resists a significant change in ph upon addition. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer is a solution that maintains the stability of a system’s ph. What Is Buffer In Botany.