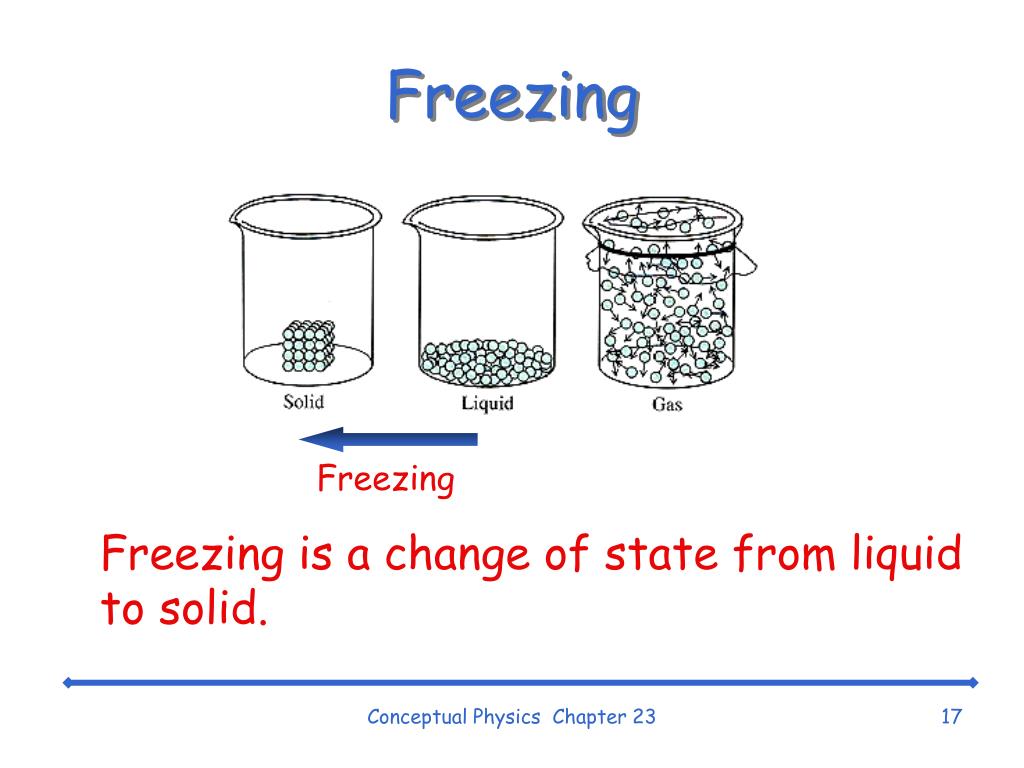
Describe Expansion Upon Freezing . The fact that water reaches a maximum density at about 4°c causes bodies of water to freeze on the top first. It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. The reason for this, simply put, is because of the structure of the lattice formed by the molecules upon freezing. When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. Upon freezing, the molecules set themselves in a very open arrangement that contains more space than the water in the liquid state. Hydrogen bonds play a crucial role in the expansion of water when it freezes. Hence, water is said to expand upon freezing and becomes less dense. Water (its volume expansion upon freezing) water is an extremely important molecule for life as we know it. When water freezes, the molecules get themselves into the most stable configurations or positions that have the minimum amount of energy in the resulting ice crystal. And so ice expands when it freezes. The fact that water expands upon freezing causes icebergs to float. This expansion upon freezing is what we refer to as the “anomalous expansion of water.” this anomaly was further studied and explained through the unique molecular structure of water. On the other hand, it As the water cools and approaches the freezing point, instead of continuing to contract, it begins to expand below 4°c.
from www.slideserve.com
Water (its volume expansion upon freezing) water is an extremely important molecule for life as we know it. Hence, water is said to expand upon freezing and becomes less dense. And so ice expands when it freezes. The fact that water reaches a maximum density at about 4°c causes bodies of water to freeze on the top first. The reason for this, simply put, is because of the structure of the lattice formed by the molecules upon freezing. When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. On the other hand, it It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. Hydrogen bonds play a crucial role in the expansion of water when it freezes. The fact that water expands upon freezing causes icebergs to float.
PPT Chapter 23 Changes of Phase PowerPoint Presentation ID779795
Describe Expansion Upon Freezing When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. Hence, water is said to expand upon freezing and becomes less dense. The reason for this, simply put, is because of the structure of the lattice formed by the molecules upon freezing. It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. And so ice expands when it freezes. As the water cools and approaches the freezing point, instead of continuing to contract, it begins to expand below 4°c. On the other hand, it The fact that water expands upon freezing causes icebergs to float. Hydrogen bonds play a crucial role in the expansion of water when it freezes. This expansion upon freezing is what we refer to as the “anomalous expansion of water.” this anomaly was further studied and explained through the unique molecular structure of water. The fact that water reaches a maximum density at about 4°c causes bodies of water to freeze on the top first. When water freezes, the molecules get themselves into the most stable configurations or positions that have the minimum amount of energy in the resulting ice crystal. When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. Upon freezing, the molecules set themselves in a very open arrangement that contains more space than the water in the liquid state. Water (its volume expansion upon freezing) water is an extremely important molecule for life as we know it.
From www.slideserve.com
PPT Temperature, Heat, and Expansion PowerPoint Presentation, free Describe Expansion Upon Freezing It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. Upon freezing, the molecules set themselves in a very open arrangement that contains more space than the water in the liquid state. As the water cools and approaches the freezing point, instead of continuing to contract, it begins. Describe Expansion Upon Freezing.
From www.slideserve.com
PPT Ch 3 The Polarity of Water and Its Properties PowerPoint Describe Expansion Upon Freezing Hence, water is said to expand upon freezing and becomes less dense. The fact that water reaches a maximum density at about 4°c causes bodies of water to freeze on the top first. On the other hand, it Water (its volume expansion upon freezing) water is an extremely important molecule for life as we know it. The reason for this,. Describe Expansion Upon Freezing.
From slideplayer.com
Biology EOC Exam Review ppt download Describe Expansion Upon Freezing The fact that water reaches a maximum density at about 4°c causes bodies of water to freeze on the top first. Upon freezing, the molecules set themselves in a very open arrangement that contains more space than the water in the liquid state. The fact that water expands upon freezing causes icebergs to float. This expansion upon freezing is what. Describe Expansion Upon Freezing.
From www.youtube.com
Change in States of matter Freezing Condensation Melting Describe Expansion Upon Freezing When water freezes, the molecules get themselves into the most stable configurations or positions that have the minimum amount of energy in the resulting ice crystal. It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. Upon freezing, the molecules set themselves in a very open arrangement that. Describe Expansion Upon Freezing.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Describe Expansion Upon Freezing When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. This expansion upon freezing is what we refer to as the “anomalous expansion of water.” this anomaly was further. Describe Expansion Upon Freezing.
From slideplayer.com
How does H2O form??. ppt download Describe Expansion Upon Freezing Hence, water is said to expand upon freezing and becomes less dense. It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. The fact that water expands upon freezing causes icebergs to float. And so ice expands when it freezes. As the water cools and approaches the freezing. Describe Expansion Upon Freezing.
From www.slideserve.com
PPT Chapters 1234 PowerPoint Presentation, free download ID6659437 Describe Expansion Upon Freezing It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. Upon freezing, the molecules set themselves in a very open arrangement that contains more space than the water in the liquid state. As the water cools and approaches the freezing point, instead of continuing to contract, it begins. Describe Expansion Upon Freezing.
From www.expii.com
Freezing — Definition & Overview Expii Describe Expansion Upon Freezing The reason for this, simply put, is because of the structure of the lattice formed by the molecules upon freezing. Hydrogen bonds play a crucial role in the expansion of water when it freezes. When water freezes, the molecules get themselves into the most stable configurations or positions that have the minimum amount of energy in the resulting ice crystal.. Describe Expansion Upon Freezing.
From ar.inspiredpencil.com
Anomalous Expansion Of Water Describe Expansion Upon Freezing Water (its volume expansion upon freezing) water is an extremely important molecule for life as we know it. Upon freezing, the molecules set themselves in a very open arrangement that contains more space than the water in the liquid state. And so ice expands when it freezes. Hence, water is said to expand upon freezing and becomes less dense. The. Describe Expansion Upon Freezing.
From slideplayer.com
Physical Science unit 3 Properities of Matter. ppt download Describe Expansion Upon Freezing When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. Hence, water is said to expand upon freezing and becomes less dense. The fact that water expands upon freezing causes icebergs to float. It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes. Describe Expansion Upon Freezing.
From slideplayer.com
Water. ppt download Describe Expansion Upon Freezing When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. When water freezes, the molecules get themselves into the most stable configurations or positions that have the minimum amount of energy in the resulting ice crystal. On the other hand, it Hydrogen bonds play a crucial role in the expansion of. Describe Expansion Upon Freezing.
From www.numerade.com
SOLVEDThe work of expansion in freezing an ice cube. At 1 atm, freeze Describe Expansion Upon Freezing The reason for this, simply put, is because of the structure of the lattice formed by the molecules upon freezing. Hydrogen bonds play a crucial role in the expansion of water when it freezes. The fact that water reaches a maximum density at about 4°c causes bodies of water to freeze on the top first. Water (its volume expansion upon. Describe Expansion Upon Freezing.
From www.slideserve.com
PPT Chapter 23 Changes of Phase PowerPoint Presentation ID779795 Describe Expansion Upon Freezing The reason for this, simply put, is because of the structure of the lattice formed by the molecules upon freezing. Hydrogen bonds play a crucial role in the expansion of water when it freezes. On the other hand, it And so ice expands when it freezes. This expansion upon freezing is what we refer to as the “anomalous expansion of. Describe Expansion Upon Freezing.
From slideplayer.com
Chapter 3 Presentation Water and the Fitness of the Environment ppt Describe Expansion Upon Freezing When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. When water freezes, the molecules get themselves into the most stable configurations or positions that have the minimum amount of energy in the resulting ice crystal. On the other hand, it The fact that water reaches a maximum density at about. Describe Expansion Upon Freezing.
From www.sciencefacts.net
Water Expansion When Freezing Describe Expansion Upon Freezing When water freezes, the molecules get themselves into the most stable configurations or positions that have the minimum amount of energy in the resulting ice crystal. This expansion upon freezing is what we refer to as the “anomalous expansion of water.” this anomaly was further studied and explained through the unique molecular structure of water. The fact that water reaches. Describe Expansion Upon Freezing.
From slideplayer.com
Water and the Fitness of the Environment ppt download Describe Expansion Upon Freezing On the other hand, it It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. When water freezes, the molecules get themselves into the most stable configurations or positions that have the minimum amount of energy in the resulting ice crystal. The fact that water reaches a maximum. Describe Expansion Upon Freezing.
From slideplayer.com
21.8 Thermal Expansion When the temperature of a substance is increased Describe Expansion Upon Freezing The reason for this, simply put, is because of the structure of the lattice formed by the molecules upon freezing. On the other hand, it As the water cools and approaches the freezing point, instead of continuing to contract, it begins to expand below 4°c. Water (its volume expansion upon freezing) water is an extremely important molecule for life as. Describe Expansion Upon Freezing.
From slideplayer.com
How Do The Properties of Water Support Life on Earth? ppt download Describe Expansion Upon Freezing Hydrogen bonds play a crucial role in the expansion of water when it freezes. It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. The fact that water expands upon freezing causes icebergs to float. Hence, water is said to expand upon freezing and becomes less dense. When. Describe Expansion Upon Freezing.
From www.worksheetsplanet.com
What is Freezing Definition of Freezing Describe Expansion Upon Freezing Water (its volume expansion upon freezing) water is an extremely important molecule for life as we know it. When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. The fact that water reaches a maximum density at about 4°c causes bodies of water to freeze on the top first. As the. Describe Expansion Upon Freezing.
From www.scribd.com
Materials of Importance Water (Its Volume Expansion Upon Freezing) PDF Describe Expansion Upon Freezing Water (its volume expansion upon freezing) water is an extremely important molecule for life as we know it. It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. When. Describe Expansion Upon Freezing.
From www.slideserve.com
PPT Water and the Fitness of the Environment PowerPoint Presentation Describe Expansion Upon Freezing It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. The fact that water expands upon freezing causes icebergs to float. And so ice expands when it freezes. As the water cools and approaches the freezing point, instead of continuing to contract, it begins to expand below 4°c.. Describe Expansion Upon Freezing.
From www.slideserve.com
PPT Different Phases of Matter PowerPoint Presentation, free download Describe Expansion Upon Freezing When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. On the other hand, it Hence, water is said to expand upon freezing and becomes less dense. Water (its volume expansion upon freezing) water is an extremely important molecule for life as we know it. As the water cools and approaches. Describe Expansion Upon Freezing.
From slideplayer.com
How does H2O form??. ppt download Describe Expansion Upon Freezing Hydrogen bonds play a crucial role in the expansion of water when it freezes. Upon freezing, the molecules set themselves in a very open arrangement that contains more space than the water in the liquid state. The fact that water expands upon freezing causes icebergs to float. Water (its volume expansion upon freezing) water is an extremely important molecule for. Describe Expansion Upon Freezing.
From slideplayer.com
August 24th , What is a hypothesis? ppt download Describe Expansion Upon Freezing Hydrogen bonds play a crucial role in the expansion of water when it freezes. As the water cools and approaches the freezing point, instead of continuing to contract, it begins to expand below 4°c. Water (its volume expansion upon freezing) water is an extremely important molecule for life as we know it. When water is in its liquid state, the. Describe Expansion Upon Freezing.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download Describe Expansion Upon Freezing When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. And so ice expands when it freezes. Water (its volume expansion upon freezing) water is an extremely important molecule for life as we know it. The fact that water reaches a maximum density at about 4°c causes bodies of water to. Describe Expansion Upon Freezing.
From www.slideserve.com
PPT Nature of Water PowerPoint Presentation, free download ID6091714 Describe Expansion Upon Freezing And so ice expands when it freezes. Hydrogen bonds play a crucial role in the expansion of water when it freezes. It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. The fact that water expands upon freezing causes icebergs to float. This expansion upon freezing is what. Describe Expansion Upon Freezing.
From www.slideserve.com
PPT Water and Water Use I PowerPoint Presentation, free download ID Describe Expansion Upon Freezing It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. This expansion upon freezing is what we refer to as the “anomalous expansion of water.” this anomaly was further studied and explained through the unique molecular structure of water. The reason for this, simply put, is because of. Describe Expansion Upon Freezing.
From studylib.net
Living with Thermal Expansion and Contraction Describe Expansion Upon Freezing It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. Upon freezing, the molecules set themselves in a very open arrangement that contains more space than the water in the liquid state. When water freezes, the molecules get themselves into the most stable configurations or positions that have. Describe Expansion Upon Freezing.
From www.chegg.com
Solved 1. Upon freezing (i.e., transforming from a liquid to Describe Expansion Upon Freezing And so ice expands when it freezes. Water (its volume expansion upon freezing) water is an extremely important molecule for life as we know it. It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. When water freezes, the molecules get themselves into the most stable configurations or. Describe Expansion Upon Freezing.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download Describe Expansion Upon Freezing When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. Upon freezing, the molecules set themselves in a very open arrangement that contains more space than the water in the liquid state. Hydrogen bonds play a crucial role in the expansion of water when it freezes. The reason for this, simply. Describe Expansion Upon Freezing.
From slideplayer.com
Water and the Fitness of the Environment ppt download Describe Expansion Upon Freezing When water freezes, the molecules get themselves into the most stable configurations or positions that have the minimum amount of energy in the resulting ice crystal. When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. As the water cools and approaches the freezing point, instead of continuing to contract, it. Describe Expansion Upon Freezing.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Describe Expansion Upon Freezing This expansion upon freezing is what we refer to as the “anomalous expansion of water.” this anomaly was further studied and explained through the unique molecular structure of water. And so ice expands when it freezes. It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. As the. Describe Expansion Upon Freezing.
From www.slideserve.com
PPT States Of Matter PowerPoint Presentation, free download ID6277780 Describe Expansion Upon Freezing As the water cools and approaches the freezing point, instead of continuing to contract, it begins to expand below 4°c. The fact that water reaches a maximum density at about 4°c causes bodies of water to freeze on the top first. Hydrogen bonds play a crucial role in the expansion of water when it freezes. On the other hand, it. Describe Expansion Upon Freezing.
From www.pinterest.com
Freezing Point Easy Science Chemistry education, Science flashcards Describe Expansion Upon Freezing It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. And so ice expands when it freezes. When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. Hence, water is said to expand upon freezing and becomes less dense.. Describe Expansion Upon Freezing.
From slideplayer.com
I can describe what happens during melting and freezing ppt download Describe Expansion Upon Freezing On the other hand, it Hence, water is said to expand upon freezing and becomes less dense. When water is in its liquid state, the molecules are moving around and constantly forming and breaking hydrogen bonds. The reason for this, simply put, is because of the structure of the lattice formed by the molecules upon freezing. The fact that water. Describe Expansion Upon Freezing.