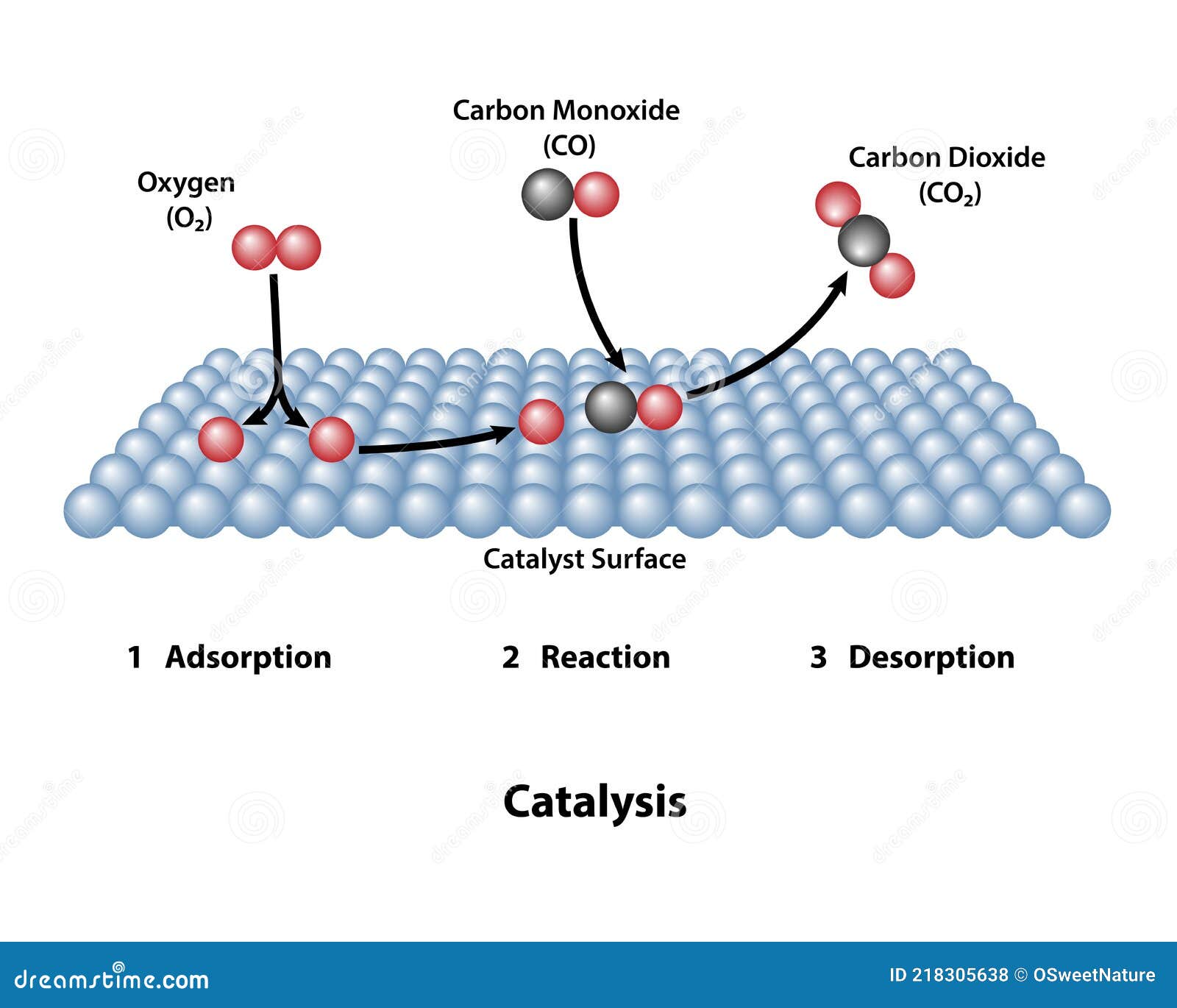
Catalyst Activity Definition . catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors.
from www.dreamstime.com
in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors.
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of
Catalyst Activity Definition catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
From dxowsfper.blob.core.windows.net
Define Catalyst Used In A Sentence at Don Peter blog Catalyst Activity Definition catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than. Catalyst Activity Definition.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Activity Definition Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. . Catalyst Activity Definition.
From www.britannica.com
Catalysis Chemistry, Classification, & Chemical Reactions Britannica Catalyst Activity Definition catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being.. Catalyst Activity Definition.
From www.youtube.com
Catalytic activity Meaning YouTube Catalyst Activity Definition catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than. Catalyst Activity Definition.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalyst Activity Definition catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being.. Catalyst Activity Definition.
From www.slideshare.net
Catalyst Catalyst Activity Definition catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. . Catalyst Activity Definition.
From www.tes.com
Catalysts lesson and experiment Teaching Resources Catalyst Activity Definition catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing. Catalyst Activity Definition.
From www.researchgate.net
Catalyst activity evaluation experimental process Download Scientific Catalyst Activity Definition Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the. Catalyst Activity Definition.
From www.slideserve.com
PPT Catalytic Reaction PowerPoint Presentation, free Catalyst Activity Definition catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which. Catalyst Activity Definition.
From www.researchgate.net
Different factors influencing the catalytic activity of the catalyst Catalyst Activity Definition catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by. Catalyst Activity Definition.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Activity Definition in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being. Catalyst Activity Definition.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Activity Definition catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors.. Catalyst Activity Definition.
From dxomuorny.blob.core.windows.net
Catalyst In Chemistry Examples at Alton Stonge blog Catalyst Activity Definition catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a. Catalyst Activity Definition.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Activity Definition catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced. Catalyst Activity Definition.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Activity Definition Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the. Catalyst Activity Definition.
From www.researchgate.net
Catalysts activity and stability (C 2 H 4 700 ppm, O 2 20, N 2 Catalyst Activity Definition in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalytic. Catalyst Activity Definition.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Activity Definition in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. catalysts allow a reaction to proceed via a pathway that has a lower activation energy. Catalyst Activity Definition.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalyst Activity Definition catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalysts. Catalyst Activity Definition.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Activity Definition catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction. Catalyst Activity Definition.
From www.worksheetsplanet.com
What is a Catalyst Definition of Catalyst Catalyst Activity Definition in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing.. Catalyst Activity Definition.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalyst Activity Definition Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is. Catalyst Activity Definition.
From www.cheric.org
Chemical Reaction (Reaction rate) Catalyst Activity Definition Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction. Catalyst Activity Definition.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalyst Activity Definition catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. Catalyst Activity Definition.
From dxostlfbq.blob.core.windows.net
Catalytic Definition Physics at Kathleen Wilson blog Catalyst Activity Definition Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. . Catalyst Activity Definition.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Catalyst Activity Definition catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. . Catalyst Activity Definition.
From www.researchgate.net
Catalytic activity comparison of prepared Different catalysts at Catalyst Activity Definition catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which is influenced by factors. catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.. Catalyst Activity Definition.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Activity Definition catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. catalyst activity refers. Catalyst Activity Definition.
From www.sliderbase.com
Enzymes. A Cell's Catalysts Presentation Biology Catalyst Activity Definition Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being.. Catalyst Activity Definition.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Activity Definition catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalytic activity. Catalyst Activity Definition.
From dxowsfper.blob.core.windows.net
Define Catalyst Used In A Sentence at Don Peter blog Catalyst Activity Definition catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of. Catalyst Activity Definition.
From www.researchgate.net
The catalyst activity as a function of time during the Catalyst Activity Definition catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. Catalytic activity refers to the ability of a catalyst to increase the rate of a chemical reaction without undergoing. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. . Catalyst Activity Definition.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalyst Activity Definition in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalytic. Catalyst Activity Definition.
From www.youtube.com
Definition of Catalyst Chemical Chemistry Class 12 YouTube Catalyst Activity Definition catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. Catalytic activity. Catalyst Activity Definition.
From www.slideshare.net
Biology 2.4 Catalyst Activity Definition catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalyst activity refers to the ability of a catalyst to convert ch4 and co2 molecules to syngas, which. Catalyst Activity Definition.
From www.sliderbase.com
Catalytic Activity Catalyst Activity Definition catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalytic activity refers to the ability of a substance, typically a catalyst, to accelerate a chemical reaction without being. catalysts. Catalyst Activity Definition.