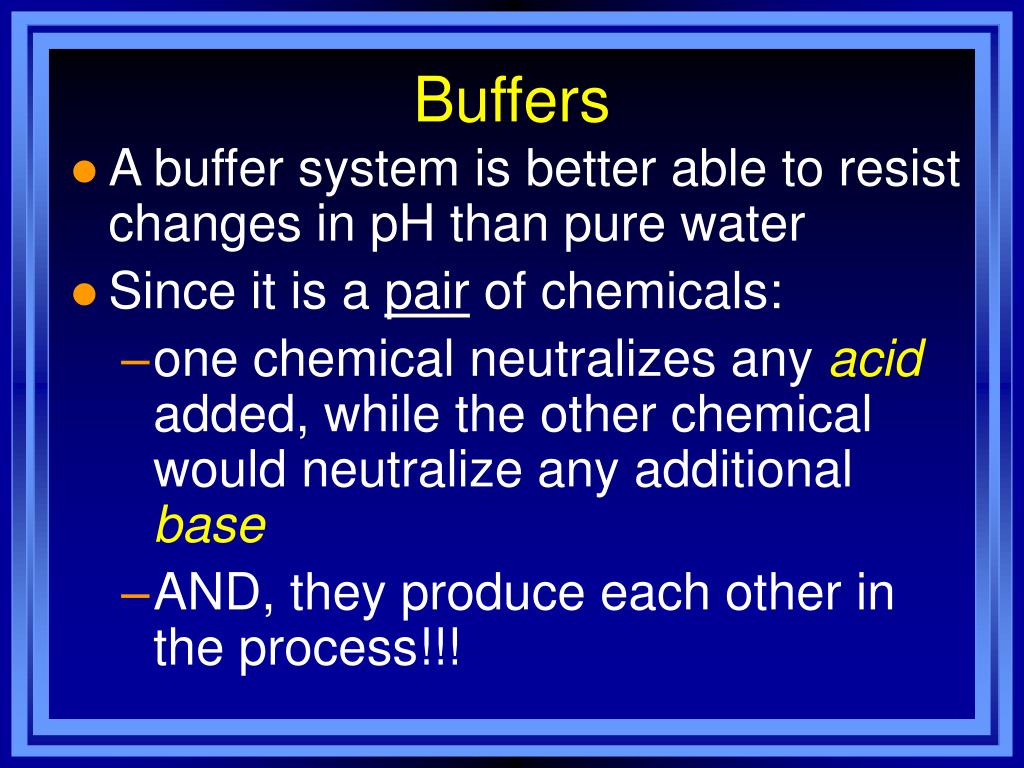
Buffers Examples Biology . buffers do so by being composed of certain pairs of solutes: what are examples of biological buffers? the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. biological buffers are compounds that help the body maintain a ph around 7.4. There are various biological buffers designed to maintain physiological ph, especially for. Either a weak acid plus its conjugate base or a weak base plus its. buffers are the key. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph.
from www.slideserve.com
buffers do so by being composed of certain pairs of solutes: Either a weak acid plus its conjugate base or a weak base plus its. There are various biological buffers designed to maintain physiological ph, especially for. what are examples of biological buffers? buffers are the key. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. biological buffers are compounds that help the body maintain a ph around 7.4.
PPT Chapter 21 “Neutralization” PowerPoint Presentation, free
Buffers Examples Biology In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. Either a weak acid plus its conjugate base or a weak base plus its. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. There are various biological buffers designed to maintain physiological ph, especially for. what are examples of biological buffers? biological buffers are compounds that help the body maintain a ph around 7.4. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers are the key. buffers do so by being composed of certain pairs of solutes:
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs Buffers Examples Biology In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. buffers are the key. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. There are various biological buffers designed to maintain physiological ph,. Buffers Examples Biology.
From www.slideserve.com
PPT Chapter 21 “Neutralization” PowerPoint Presentation, free Buffers Examples Biology what are examples of biological buffers? the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. buffers do so by being composed of certain pairs of solutes: the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. biological buffers are compounds that. Buffers Examples Biology.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Buffers Examples Biology buffers are the key. biological buffers are compounds that help the body maintain a ph around 7.4. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. There are various biological buffers designed to maintain physiological ph, especially for. In this article, you will be able to. Buffers Examples Biology.
From slideplayer.com
Chem. 1B 9/24 Lecture. Announcements I Exam 1 On Oct. 1 here Buffers Examples Biology There are various biological buffers designed to maintain physiological ph, especially for. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. what are examples of biological buffers? buffers are the key. In this article, you will be able to describe what a buffer is, why buffers are important, and. Buffers Examples Biology.
From www.pinterest.com
Pin on Download Buffers Examples Biology Either a weak acid plus its conjugate base or a weak base plus its. what are examples of biological buffers? buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. buffers do so by being composed of certain pairs of solutes: buffers are the key. . Buffers Examples Biology.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Buffers Examples Biology buffers are the key. buffers do so by being composed of certain pairs of solutes: the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. what are examples of biological buffers? In this article, you will be able to describe what a buffer is, why buffers are important, and how. Buffers Examples Biology.
From exygziwff.blob.core.windows.net
Examples Of Buffers In Biological Systems at Leatha Boles blog Buffers Examples Biology biological buffers are compounds that help the body maintain a ph around 7.4. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium. Buffers Examples Biology.
From ar.inspiredpencil.com
Buffer Chemistry Example Buffers Examples Biology There are various biological buffers designed to maintain physiological ph, especially for. what are examples of biological buffers? buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. biological buffers are compounds that help the body maintain a ph around 7.4. In this article, you will be. Buffers Examples Biology.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching Buffers Examples Biology the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. In this article, you. Buffers Examples Biology.
From www.youtube.com
Buffers and pH Biology YouTube Buffers Examples Biology Either a weak acid plus its conjugate base or a weak base plus its. buffers are the key. biological buffers are compounds that help the body maintain a ph around 7.4. what are examples of biological buffers? the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the buffer. Buffers Examples Biology.
From exygziwff.blob.core.windows.net
Examples Of Buffers In Biological Systems at Leatha Boles blog Buffers Examples Biology biological buffers are compounds that help the body maintain a ph around 7.4. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. buffers are the key. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance. Buffers Examples Biology.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Buffers Examples Biology biological buffers are compounds that help the body maintain a ph around 7.4. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Either a weak acid plus its conjugate base or a weak base plus its. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which. Buffers Examples Biology.
From www.slideshare.net
Buffers in chemical analysis, types of buffers Buffers Examples Biology buffers are the key. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants. Buffers Examples Biology.
From thechemistrynotes.com
Buffer Solution Types, Properties, and Uses Buffers Examples Biology buffers do so by being composed of certain pairs of solutes: the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Either a weak acid plus its conjugate base or a weak base plus its. There are various biological buffers designed to maintain physiological ph, especially for. buffers and how they. Buffers Examples Biology.
From giobnpmka.blob.core.windows.net
Buffer Definition Biology Sentence at Jeff Everett blog Buffers Examples Biology buffers do so by being composed of certain pairs of solutes: what are examples of biological buffers? There are various biological buffers designed to maintain physiological ph, especially for. Either a weak acid plus its conjugate base or a weak base plus its. buffers and how they control hydrogen ion concentrations, a brief explanation of the role. Buffers Examples Biology.
From dandkmotorsports.com
Hepes Buffer Recipe Ph 7 2 Dandk Organizer Buffers Examples Biology There are various biological buffers designed to maintain physiological ph, especially for. buffers are the key. biological buffers are compounds that help the body maintain a ph around 7.4. what are examples of biological buffers? the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. buffers and how they. Buffers Examples Biology.
From www.pinterest.co.uk
Buffer Easy Science Chemistry education, Study chemistry, Chemistry Buffers Examples Biology the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. biological buffers are compounds that help the body maintain a ph around 7.4. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. Either a weak acid plus its conjugate. Buffers Examples Biology.
From fr.slideshare.net
Buffer solution Buffers Examples Biology the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. buffers are the key. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood. Buffers Examples Biology.
From www.slideserve.com
PPT Buffered Solutions PowerPoint Presentation, free download ID Buffers Examples Biology what are examples of biological buffers? Either a weak acid plus its conjugate base or a weak base plus its. There are various biological buffers designed to maintain physiological ph, especially for. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. buffers and how they control hydrogen ion concentrations, a. Buffers Examples Biology.
From karen-blogewing.blogspot.com
Find Which Acid Is Best for a Ph 4 Buffer Buffers Examples Biology the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. buffers do so by being composed of certain pairs of solutes: There are various biological buffers designed to maintain physiological ph, especially for. what are examples of biological buffers? buffers and how they control hydrogen ion concentrations, a brief explanation. Buffers Examples Biology.
From www.youtube.com
WCLN Buffers Biology YouTube Buffers Examples Biology what are examples of biological buffers? the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. In this article, you will be able to describe what a buffer is, why. Buffers Examples Biology.
From animalia-life.club
Phosphate Buffer System Equation Buffers Examples Biology buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. Either a weak acid plus its conjugate base or a weak base plus its. buffers are the key. There are various biological buffers designed to maintain physiological ph, especially for. buffers do so by being composed of. Buffers Examples Biology.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Buffers Examples Biology buffers are the key. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. what are examples of biological buffers? the buffer systems. Buffers Examples Biology.
From www.researchgate.net
Examples of buffer compounds divided into accepted physiological Buffers Examples Biology buffers are the key. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Either a weak acid plus its conjugate base or a weak base plus its. There are various biological buffers designed to maintain physiological ph, especially for. biological buffers are compounds that help the body maintain a. Buffers Examples Biology.
From fyoapmfvg.blob.core.windows.net
Is Salt A Buffer at Guy Bower blog Buffers Examples Biology the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. what are examples of biological buffers? In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. biological buffers are compounds that help the. Buffers Examples Biology.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID6787517 Buffers Examples Biology buffers are the key. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. biological buffers are compounds that help the body maintain a ph around 7.4. the. Buffers Examples Biology.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high Buffers Examples Biology Either a weak acid plus its conjugate base or a weak base plus its. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. biological buffers are compounds that help the body maintain a ph around 7.4. There are various biological buffers designed to maintain physiological ph, especially for. the. Buffers Examples Biology.
From exyfnmepw.blob.core.windows.net
Common Molecular Biology Buffers at Donna Davis blog Buffers Examples Biology buffers do so by being composed of certain pairs of solutes: buffers are the key. what are examples of biological buffers? In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. There are various biological buffers designed to maintain physiological. Buffers Examples Biology.
From deporecipe.co
10x Protein Running Buffer Recipe Deporecipe.co Buffers Examples Biology There are various biological buffers designed to maintain physiological ph, especially for. buffers are the key. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. what are examples of biological buffers? Either a weak acid plus its conjugate base or a weak base plus its. buffers and how. Buffers Examples Biology.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Buffers Examples Biology the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers do so by being composed of certain pairs of solutes: what are examples of biological buffers? Either a weak acid plus its. Buffers Examples Biology.
From facts.net
13 Enigmatic Facts About Buffer Capacity Buffers Examples Biology buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. There are various biological buffers designed to maintain physiological ph, especially for. what are examples of biological buffers? the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. In this article,. Buffers Examples Biology.
From fyoqfobay.blob.core.windows.net
Biological Buffer System Examples at Michele Huff blog Buffers Examples Biology buffers do so by being composed of certain pairs of solutes: the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. In this article, you will be able to describe what. Buffers Examples Biology.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies Buffers Examples Biology Either a weak acid plus its conjugate base or a weak base plus its. buffers are the key. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. There are various biological buffers designed to maintain physiological ph, especially for. buffers do so by being composed of certain pairs of solutes:. Buffers Examples Biology.
From studylib.net
Buffer Solutions Buffers Examples Biology buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. There are various biological buffers designed to maintain physiological ph, especially for. Either a weak acid plus its conjugate base or a weak base plus its. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and. Buffers Examples Biology.
From exyfnmepw.blob.core.windows.net
Common Molecular Biology Buffers at Donna Davis blog Buffers Examples Biology biological buffers are compounds that help the body maintain a ph around 7.4. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. In this article, you will be able to. Buffers Examples Biology.