Types Of Bonding Interactions . chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. Ionic and covalent bonds are strong. There are 4 primary types of chemical. the type of chemical bonds formed varies in strength and properties. Chemical bonding tends to be of two types; all models of chemical bonding have three common features: Since electrons are negatively charged, an atom that loses. Covalent, in which electrons are shared between atoms, and ionic. there are four types of bonds or interactions: ions form when atoms gain or lose electrons. A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or. Ionic, covalent, hydrogen bonds, and van der waals interactions. types of chemical bonds: Atoms form bonds because the products are more stable.
from www3.nd.edu
there are four types of bonds or interactions: There are 4 primary types of chemical. Since electrons are negatively charged, an atom that loses. all models of chemical bonding have three common features: the type of chemical bonds formed varies in strength and properties. A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or. Ionic, covalent, hydrogen bonds, and van der waals interactions. Chemical bonding tends to be of two types; chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. Ionic and covalent bonds are strong.
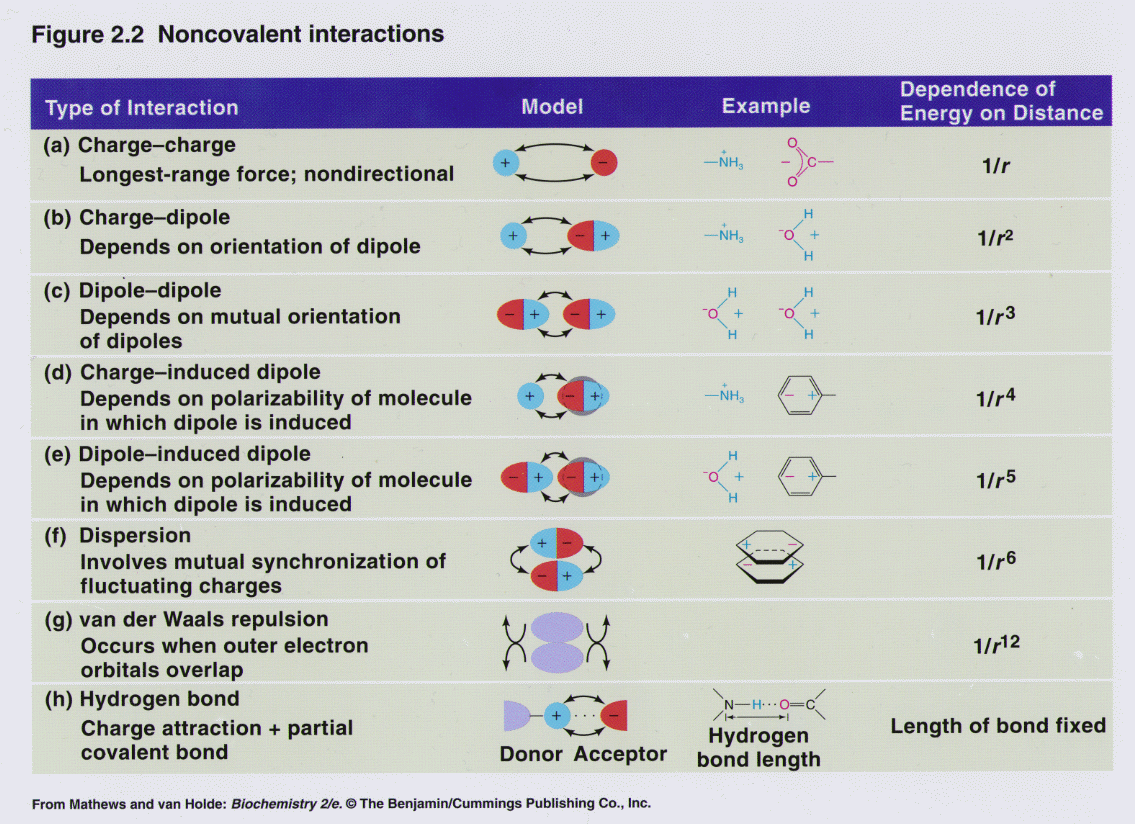
Noncovalent Interactions
Types Of Bonding Interactions Ionic and covalent bonds are strong. there are four types of bonds or interactions: Since electrons are negatively charged, an atom that loses. Ionic, covalent, hydrogen bonds, and van der waals interactions. types of chemical bonds: the type of chemical bonds formed varies in strength and properties. A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or. Chemical bonding tends to be of two types; chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. ions form when atoms gain or lose electrons. There are 4 primary types of chemical. Atoms form bonds because the products are more stable. Covalent, in which electrons are shared between atoms, and ionic. all models of chemical bonding have three common features: Ionic and covalent bonds are strong.
From www.biologyexams4u.com
Biology Exams 4 U Types Of Bonding Interactions ions form when atoms gain or lose electrons. Ionic and covalent bonds are strong. There are 4 primary types of chemical. Covalent, in which electrons are shared between atoms, and ionic. all models of chemical bonding have three common features: types of chemical bonds: chemical bonding, any of the interactions that account for the association of. Types Of Bonding Interactions.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Types Of Bonding Interactions Atoms form bonds because the products are more stable. A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or. Ionic, covalent, hydrogen bonds, and van der waals interactions. the type of chemical bonds formed varies in strength and properties. Chemical bonding tends to be of two types; chemical. Types Of Bonding Interactions.
From www.chemistrylearner.com
Hydrogen Bond Definition, Types, and Examples Types Of Bonding Interactions chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. Ionic, covalent, hydrogen bonds, and van der waals interactions. all models of chemical bonding have three common features: Ionic and covalent bonds are strong. Covalent, in which electrons are shared between atoms, and ionic. Atoms form bonds because. Types Of Bonding Interactions.
From www.vectorstock.com
Types of chemical bonding Royalty Free Vector Image Types Of Bonding Interactions types of chemical bonds: chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or. all models of chemical bonding have three common features: Chemical bonding tends to. Types Of Bonding Interactions.
From www.expii.com
Van der Waals Forces — Definition & Overview Expii Types Of Bonding Interactions Since electrons are negatively charged, an atom that loses. types of chemical bonds: Ionic and covalent bonds are strong. Chemical bonding tends to be of two types; the type of chemical bonds formed varies in strength and properties. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other. Types Of Bonding Interactions.
From www.slideserve.com
PPT Amino Acids PowerPoint Presentation, free download ID2219621 Types Of Bonding Interactions types of chemical bonds: the type of chemical bonds formed varies in strength and properties. Since electrons are negatively charged, an atom that loses. Ionic and covalent bonds are strong. Chemical bonding tends to be of two types; Ionic, covalent, hydrogen bonds, and van der waals interactions. ions form when atoms gain or lose electrons. There are. Types Of Bonding Interactions.
From www.researchgate.net
The optimized geometries of amino acids and the structures of four Types Of Bonding Interactions Covalent, in which electrons are shared between atoms, and ionic. types of chemical bonds: Atoms form bonds because the products are more stable. Chemical bonding tends to be of two types; A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or. all models of chemical bonding have three. Types Of Bonding Interactions.
From www.researchgate.net
Different intermolecular interaction types and their energies. Taken Types Of Bonding Interactions Chemical bonding tends to be of two types; Covalent, in which electrons are shared between atoms, and ionic. Atoms form bonds because the products are more stable. there are four types of bonds or interactions: types of chemical bonds: Ionic and covalent bonds are strong. Ionic, covalent, hydrogen bonds, and van der waals interactions. A weak bond in. Types Of Bonding Interactions.
From www.researchgate.net
Distance dependencies of noncovalent interactions Download Table Types Of Bonding Interactions chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. Atoms form bonds because the products are more stable. ions form when atoms gain or lose electrons. There are 4 primary types of chemical. Chemical bonding tends to be of two types; Ionic and covalent bonds are strong.. Types Of Bonding Interactions.
From youtube.com
Bonds in Protein Structure YouTube Types Of Bonding Interactions Ionic and covalent bonds are strong. the type of chemical bonds formed varies in strength and properties. there are four types of bonds or interactions: Ionic, covalent, hydrogen bonds, and van der waals interactions. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. Atoms form bonds. Types Of Bonding Interactions.
From www.phdnest.com
Covalent Bond Meaning, Types, Properties, Examples PhD Nest Types Of Bonding Interactions types of chemical bonds: Ionic, covalent, hydrogen bonds, and van der waals interactions. Covalent, in which electrons are shared between atoms, and ionic. There are 4 primary types of chemical. the type of chemical bonds formed varies in strength and properties. Ionic and covalent bonds are strong. Since electrons are negatively charged, an atom that loses. Atoms form. Types Of Bonding Interactions.
From printyourmoment.nl
hydrogen bond. chemistry lesson. Infographic. hydrogen bonds between Types Of Bonding Interactions there are four types of bonds or interactions: types of chemical bonds: ions form when atoms gain or lose electrons. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. the type of chemical bonds formed varies in strength and properties. Covalent, in which electrons. Types Of Bonding Interactions.
From sciencenotes.org
Intermolecular Forces in Chemistry Types Of Bonding Interactions all models of chemical bonding have three common features: Chemical bonding tends to be of two types; ions form when atoms gain or lose electrons. Covalent, in which electrons are shared between atoms, and ionic. the type of chemical bonds formed varies in strength and properties. Ionic and covalent bonds are strong. There are 4 primary types. Types Of Bonding Interactions.
From testbook.com
Atomic Structure and chemical bonds MCQ [Free PDF] Objective Question Types Of Bonding Interactions Chemical bonding tends to be of two types; chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. all models of chemical bonding have three common features: types of chemical bonds: Since electrons are negatively charged, an atom that loses. the type of chemical bonds formed. Types Of Bonding Interactions.
From byjus.com
Which of the following are responsible for tertiary structure of Types Of Bonding Interactions Atoms form bonds because the products are more stable. Covalent, in which electrons are shared between atoms, and ionic. ions form when atoms gain or lose electrons. types of chemical bonds: Ionic and covalent bonds are strong. Chemical bonding tends to be of two types; chemical bonding, any of the interactions that account for the association of. Types Of Bonding Interactions.
From www.chemistrylearner.com
Van der Waals Forces (Bond) Definition, Examples, & Diagrams Types Of Bonding Interactions Since electrons are negatively charged, an atom that loses. There are 4 primary types of chemical. the type of chemical bonds formed varies in strength and properties. Ionic and covalent bonds are strong. ions form when atoms gain or lose electrons. A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom. Types Of Bonding Interactions.
From inovasibiologi.com
Ikatan Kimia INOVASI BIOLOGI Types Of Bonding Interactions types of chemical bonds: A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or. Atoms form bonds because the products are more stable. There are 4 primary types of chemical. all models of chemical bonding have three common features: Ionic and covalent bonds are strong. there are. Types Of Bonding Interactions.
From www.slideserve.com
PPT Protein structural element PowerPoint Presentation, free download Types Of Bonding Interactions Ionic, covalent, hydrogen bonds, and van der waals interactions. types of chemical bonds: A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or. Atoms form bonds because the products are more stable. Covalent, in which electrons are shared between atoms, and ionic. there are four types of bonds. Types Of Bonding Interactions.
From www.chegg.com
Solved Identify the type of bond(s) formed from the Types Of Bonding Interactions the type of chemical bonds formed varies in strength and properties. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or. all models of chemical bonding have. Types Of Bonding Interactions.
From www.slideserve.com
PPT Interatomic Bonding PowerPoint Presentation, free download ID Types Of Bonding Interactions the type of chemical bonds formed varies in strength and properties. Chemical bonding tends to be of two types; Atoms form bonds because the products are more stable. A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or. chemical bonding, any of the interactions that account for the. Types Of Bonding Interactions.
From en.wikipedia.org
Ionic bonding Wikipedia Types Of Bonding Interactions Chemical bonding tends to be of two types; Since electrons are negatively charged, an atom that loses. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. there are four types of bonds or interactions: types of chemical bonds: Atoms form bonds because the products are more. Types Of Bonding Interactions.
From quizlet.com
intermolecular vs. intramolecular forces Diagram Quizlet Types Of Bonding Interactions Ionic, covalent, hydrogen bonds, and van der waals interactions. Covalent, in which electrons are shared between atoms, and ionic. Atoms form bonds because the products are more stable. There are 4 primary types of chemical. there are four types of bonds or interactions: A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative. Types Of Bonding Interactions.
From www3.nd.edu
Noncovalent Interactions Types Of Bonding Interactions there are four types of bonds or interactions: types of chemical bonds: Ionic, covalent, hydrogen bonds, and van der waals interactions. Chemical bonding tends to be of two types; A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or. the type of chemical bonds formed varies in. Types Of Bonding Interactions.
From www.researchgate.net
Positions and types of chemical bond interactions. (A) RT_Kaempferitrin Types Of Bonding Interactions Covalent, in which electrons are shared between atoms, and ionic. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. Chemical bonding tends to be of two types; there are four types of bonds or interactions: all models of chemical bonding have three common features: types. Types Of Bonding Interactions.
From www.britannica.com
Metallic bond Properties, Examples, & Explanation Britannica Types Of Bonding Interactions Ionic and covalent bonds are strong. Chemical bonding tends to be of two types; A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or. There are 4 primary types of chemical. types of chemical bonds: the type of chemical bonds formed varies in strength and properties. Covalent, in. Types Of Bonding Interactions.
From www.pearson.com
Introduction to Chemical Bonding Video Tutorials & Practice Problems Types Of Bonding Interactions Ionic, covalent, hydrogen bonds, and van der waals interactions. Ionic and covalent bonds are strong. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or. types of chemical. Types Of Bonding Interactions.
From alchetron.com
Non covalent interactions Alchetron, the free social encyclopedia Types Of Bonding Interactions Covalent, in which electrons are shared between atoms, and ionic. all models of chemical bonding have three common features: there are four types of bonds or interactions: Chemical bonding tends to be of two types; ions form when atoms gain or lose electrons. Atoms form bonds because the products are more stable. There are 4 primary types. Types Of Bonding Interactions.
From www.expii.com
Hydrogen Bonds — Overview & Examples Expii Types Of Bonding Interactions ions form when atoms gain or lose electrons. there are four types of bonds or interactions: A weak bond in which a hydrogen atom in one molecule is attracted to an electronegative atom (usually nitrogen or. types of chemical bonds: chemical bonding, any of the interactions that account for the association of atoms into molecules, ions,. Types Of Bonding Interactions.
From www.cambridgemedchemconsulting.com
Molecular Interactions Cambridge MedChem Consulting Types Of Bonding Interactions the type of chemical bonds formed varies in strength and properties. Chemical bonding tends to be of two types; Atoms form bonds because the products are more stable. There are 4 primary types of chemical. types of chemical bonds: Ionic and covalent bonds are strong. Covalent, in which electrons are shared between atoms, and ionic. Since electrons are. Types Of Bonding Interactions.
From www.slideserve.com
PPT Types of noncovalent interactions PowerPoint Presentation, free Types Of Bonding Interactions Ionic, covalent, hydrogen bonds, and van der waals interactions. Atoms form bonds because the products are more stable. Ionic and covalent bonds are strong. Since electrons are negatively charged, an atom that loses. the type of chemical bonds formed varies in strength and properties. A weak bond in which a hydrogen atom in one molecule is attracted to an. Types Of Bonding Interactions.
From www.numerade.com
Noncovalent interactions While the primary structure of TABLE 25 Four Types Of Bonding Interactions Ionic and covalent bonds are strong. there are four types of bonds or interactions: Since electrons are negatively charged, an atom that loses. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. the type of chemical bonds formed varies in strength and properties. A weak bond. Types Of Bonding Interactions.
From www.britannica.com
crystal Types of bonds Britannica Types Of Bonding Interactions ions form when atoms gain or lose electrons. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. There are 4 primary types of chemical. there are four types of bonds or interactions: all models of chemical bonding have three common features: Covalent, in which electrons. Types Of Bonding Interactions.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.3.15 Inter & Intramolecular Forces翰林国际教育 Types Of Bonding Interactions Ionic and covalent bonds are strong. types of chemical bonds: chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. Since electrons are negatively charged, an atom that loses. Atoms form bonds because the products are more stable. there are four types of bonds or interactions: Chemical. Types Of Bonding Interactions.
From xjfuxnexry.blogspot.com
Three Types Of Chemical Bonds, Covalent Bond Definition Types Polar And Types Of Bonding Interactions ions form when atoms gain or lose electrons. the type of chemical bonds formed varies in strength and properties. Since electrons are negatively charged, an atom that loses. types of chemical bonds: there are four types of bonds or interactions: chemical bonding, any of the interactions that account for the association of atoms into molecules,. Types Of Bonding Interactions.
From www.researchgate.net
Types of interfacial bonding a molecular inter diffusion, b Types Of Bonding Interactions chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable. Ionic and covalent bonds are strong. Atoms form bonds because the products are more stable. ions form when atoms gain or lose electrons. all models of chemical bonding have three common features: A weak bond in which. Types Of Bonding Interactions.