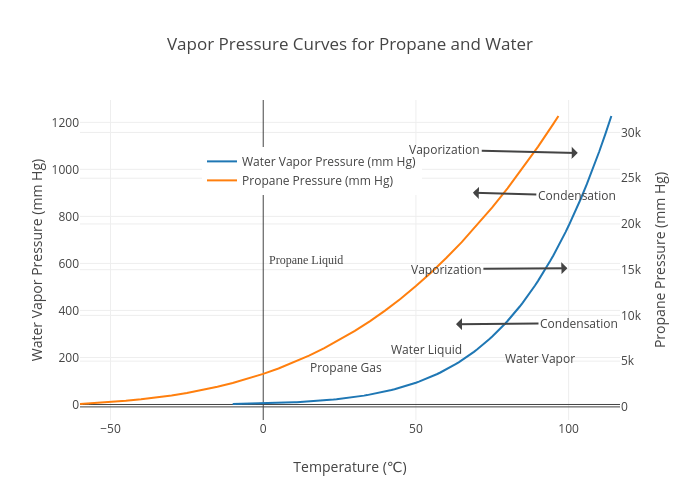
What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C . Through this graph, one can find the approximate. At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially greater than the pressure at 80.0°c, as we would expect. 【solved】click here to get an answer to your question : You can apply the ideal gas law for every gas at a density low enough to prevent the emergence of strong intermolecular forces. Water freezes at _____ k. What is the approximate vapor pressure when the gas condenses at 80°c? Vapor pressure is the pressure exerted by the vapor molecules of a substance in a closed system. 400 200 760 5 the theoretical zero volume temperature of a gas the. 1 the vapor pressure of a liquid is the pressure at which its gas phase is in equilibrium with its liquid phase at a given temperature. At 8 0 ∘ c 80^{\circ }c What is the approximate vapor pressure when the gas condenses at 80 °c? It occurs at equilibrium, i.e., when the. The _____ vapor pressure at high altitudes causes a liquid to boil at a _____ temperature. The given image of the graph explains the relation between the pressure and the temperature of the substance.
from chart-studio.plotly.com
Water freezes at _____ k. It occurs at equilibrium, i.e., when the. 【solved】click here to get an answer to your question : The given image of the graph explains the relation between the pressure and the temperature of the substance. What is the approximate vapor pressure when the gas condenses at 80 °c? At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially greater than the pressure at 80.0°c, as we would expect. At 8 0 ∘ c 80^{\circ }c 1 the vapor pressure of a liquid is the pressure at which its gas phase is in equilibrium with its liquid phase at a given temperature. The _____ vapor pressure at high altitudes causes a liquid to boil at a _____ temperature. 400 200 760 5 the theoretical zero volume temperature of a gas the.
Vapor Pressure Curves for Propane and Water line chart made by
What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C Through this graph, one can find the approximate. What is the approximate vapor pressure when the gas condenses at 80 °c? The given image of the graph explains the relation between the pressure and the temperature of the substance. What is the approximate vapor pressure when the gas condenses at 80°c? Water freezes at _____ k. At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially greater than the pressure at 80.0°c, as we would expect. It occurs at equilibrium, i.e., when the. 1 the vapor pressure of a liquid is the pressure at which its gas phase is in equilibrium with its liquid phase at a given temperature. 【solved】click here to get an answer to your question : You can apply the ideal gas law for every gas at a density low enough to prevent the emergence of strong intermolecular forces. Through this graph, one can find the approximate. 400 200 760 5 the theoretical zero volume temperature of a gas the. The _____ vapor pressure at high altitudes causes a liquid to boil at a _____ temperature. At 8 0 ∘ c 80^{\circ }c Vapor pressure is the pressure exerted by the vapor molecules of a substance in a closed system.
From www.youtube.com
Refrigeration Cycle Example YouTube What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C At 8 0 ∘ c 80^{\circ }c Through this graph, one can find the approximate. It occurs at equilibrium, i.e., when the. 1 the vapor pressure of a liquid is the pressure at which its gas phase is in equilibrium with its liquid phase at a given temperature. What is the approximate vapor pressure when the gas condenses at 80. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.studocu.com
Vapor pressure and its relationship with intermolecular forces The What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially greater than the pressure at 80.0°c, as we would expect. It occurs at equilibrium, i.e., when the. Vapor pressure is the pressure exerted by the vapor molecules of a substance in a closed system. The _____ vapor pressure at high altitudes causes a liquid to boil at a. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.chegg.com
Solved 3. [2 Points). The graph below gives the vapor What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially greater than the pressure at 80.0°c, as we would expect. The given image of the graph explains the relation between the pressure and the temperature of the substance. What is the approximate vapor pressure when the gas condenses at 80 °c? What is the approximate vapor pressure when. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C What is the approximate vapor pressure when the gas condenses at 80°c? The given image of the graph explains the relation between the pressure and the temperature of the substance. Through this graph, one can find the approximate. It occurs at equilibrium, i.e., when the. Vapor pressure is the pressure exerted by the vapor molecules of a substance in a. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.youtube.com
06 Ch 12 Vaporization, Condensation, and Vapor Pressure YouTube What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C At 8 0 ∘ c 80^{\circ }c You can apply the ideal gas law for every gas at a density low enough to prevent the emergence of strong intermolecular forces. Water freezes at _____ k. 【solved】click here to get an answer to your question : It occurs at equilibrium, i.e., when the. The _____ vapor pressure at high altitudes causes. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C The _____ vapor pressure at high altitudes causes a liquid to boil at a _____ temperature. What is the approximate vapor pressure when the gas condenses at 80°c? 【solved】click here to get an answer to your question : Through this graph, one can find the approximate. You can apply the ideal gas law for every gas at a density low. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.slideserve.com
PPT Chapter 23 Changes of Phase PowerPoint Presentation ID779795 What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C The given image of the graph explains the relation between the pressure and the temperature of the substance. Water freezes at _____ k. You can apply the ideal gas law for every gas at a density low enough to prevent the emergence of strong intermolecular forces. 400 200 760 5 the theoretical zero volume temperature of a gas the. Vapor. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From mavink.com
Hydrocarbon Vapor Pressure Chart What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C What is the approximate vapor pressure when the gas condenses at 80°c? 400 200 760 5 the theoretical zero volume temperature of a gas the. It occurs at equilibrium, i.e., when the. 1 the vapor pressure of a liquid is the pressure at which its gas phase is in equilibrium with its liquid phase at a given temperature. At 160°c,. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.trafag.com
Gas density monitoring with reference gas comparison What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C You can apply the ideal gas law for every gas at a density low enough to prevent the emergence of strong intermolecular forces. 400 200 760 5 the theoretical zero volume temperature of a gas the. Vapor pressure is the pressure exerted by the vapor molecules of a substance in a closed system. What is the approximate vapor pressure when. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.chegg.com
Solved What is the approximate vapor pressure for liquid What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C You can apply the ideal gas law for every gas at a density low enough to prevent the emergence of strong intermolecular forces. The _____ vapor pressure at high altitudes causes a liquid to boil at a _____ temperature. Water freezes at _____ k. At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially greater than the pressure. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.chegg.com
Solved In the chart are approximate vapor What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C You can apply the ideal gas law for every gas at a density low enough to prevent the emergence of strong intermolecular forces. 400 200 760 5 the theoretical zero volume temperature of a gas the. The given image of the graph explains the relation between the pressure and the temperature of the substance. What is the approximate vapor pressure. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.chegg.com
Solved The following graph indicates the vapor pressure of a What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C What is the approximate vapor pressure when the gas condenses at 80 °c? At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially greater than the pressure at 80.0°c, as we would expect. You can apply the ideal gas law for every gas at a density low enough to prevent the emergence of strong intermolecular forces. 400 200. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.chegg.com
Solved Figure 1 1 of 1 > 800 700 600 500 Vapor pressure (mm What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C At 8 0 ∘ c 80^{\circ }c What is the approximate vapor pressure when the gas condenses at 80°c? Water freezes at _____ k. It occurs at equilibrium, i.e., when the. 【solved】click here to get an answer to your question : At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially greater than the pressure at 80.0°c, as. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From courses.lumenlearning.com
Stoichiometry of Gases CHEM 1305 Introductory Chemistry What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C What is the approximate vapor pressure when the gas condenses at 80°c? 【solved】click here to get an answer to your question : The given image of the graph explains the relation between the pressure and the temperature of the substance. At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially greater than the pressure at 80.0°c, as we. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From webmis.highland.cc.il.us
Vapor Pressure What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C The _____ vapor pressure at high altitudes causes a liquid to boil at a _____ temperature. What is the approximate vapor pressure when the gas condenses at 80 °c? At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially greater than the pressure at 80.0°c, as we would expect. You can apply the ideal gas law for every. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.youtube.com
What is vapor pressure what is the vapor pressure of liquids What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C 400 200 760 5 the theoretical zero volume temperature of a gas the. Water freezes at _____ k. What is the approximate vapor pressure when the gas condenses at 80°c? The _____ vapor pressure at high altitudes causes a liquid to boil at a _____ temperature. At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially greater than. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.numerade.com
SOLVED Use the vapor pressure curves illustrated here to answer the What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C 400 200 760 5 the theoretical zero volume temperature of a gas the. 1 the vapor pressure of a liquid is the pressure at which its gas phase is in equilibrium with its liquid phase at a given temperature. The _____ vapor pressure at high altitudes causes a liquid to boil at a _____ temperature. What is the approximate vapor. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.numerade.com
SOLVEDIn an experiment, 5.00 L of N2 is saturated with water vapor at What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C The given image of the graph explains the relation between the pressure and the temperature of the substance. It occurs at equilibrium, i.e., when the. Vapor pressure is the pressure exerted by the vapor molecules of a substance in a closed system. What is the approximate vapor pressure when the gas condenses at 80°c? 1 the vapor pressure of a. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.slideserve.com
PPT Vapor Pressure and Changes of State PowerPoint Presentation, free What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C Vapor pressure is the pressure exerted by the vapor molecules of a substance in a closed system. It occurs at equilibrium, i.e., when the. You can apply the ideal gas law for every gas at a density low enough to prevent the emergence of strong intermolecular forces. Through this graph, one can find the approximate. 1 the vapor pressure of. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.numerade.com
SOLVEDWhat is the effect of a nonvolatile solute on the vapor pressure What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C The given image of the graph explains the relation between the pressure and the temperature of the substance. 1 the vapor pressure of a liquid is the pressure at which its gas phase is in equilibrium with its liquid phase at a given temperature. 400 200 760 5 the theoretical zero volume temperature of a gas the. 【solved】click here to. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From saylordotorg.github.io
Properties of Liquids What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C The _____ vapor pressure at high altitudes causes a liquid to boil at a _____ temperature. At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially greater than the pressure at 80.0°c, as we would expect. 1 the vapor pressure of a liquid is the pressure at which its gas phase is in equilibrium with its liquid phase. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.numerade.com
SOLVED Saturated steam at atmospheric pressure condenses on a 2 m high What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C What is the approximate vapor pressure when the gas condenses at 80°c? At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially greater than the pressure at 80.0°c, as we would expect. You can apply the ideal gas law for every gas at a density low enough to prevent the emergence of strong intermolecular forces. Through this graph,. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From chart-studio.plotly.com
Vapor Pressure Curves for Propane and Water line chart made by What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C 1 the vapor pressure of a liquid is the pressure at which its gas phase is in equilibrium with its liquid phase at a given temperature. What is the approximate vapor pressure when the gas condenses at 80 °c? You can apply the ideal gas law for every gas at a density low enough to prevent the emergence of strong. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.slideserve.com
PPT Chapter 7 Aerosols, Sprays and Inhalations PowerPoint What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C Through this graph, one can find the approximate. 400 200 760 5 the theoretical zero volume temperature of a gas the. You can apply the ideal gas law for every gas at a density low enough to prevent the emergence of strong intermolecular forces. Vapor pressure is the pressure exerted by the vapor molecules of a substance in a closed. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From hvacrschool.com
vapor pressure Archives HVAC School What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C Vapor pressure is the pressure exerted by the vapor molecules of a substance in a closed system. It occurs at equilibrium, i.e., when the. At 8 0 ∘ c 80^{\circ }c What is the approximate vapor pressure when the gas condenses at 80 °c? 【solved】click here to get an answer to your question : The _____ vapor pressure at high. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From flatworldknowledge.lardbucket.org
Vapor Pressure What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C 1 the vapor pressure of a liquid is the pressure at which its gas phase is in equilibrium with its liquid phase at a given temperature. It occurs at equilibrium, i.e., when the. 【solved】click here to get an answer to your question : The given image of the graph explains the relation between the pressure and the temperature of the. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.numerade.com
SOLVED This graph shows how three liquids' vapor pressure changes with What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C You can apply the ideal gas law for every gas at a density low enough to prevent the emergence of strong intermolecular forces. Water freezes at _____ k. What is the approximate vapor pressure when the gas condenses at 80 °c? Through this graph, one can find the approximate. At 160°c, liquid hg has a vapor pressure of 4.21 torr,. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.youtube.com
Heat of Vaporization from Vapor Pressure (Example) YouTube What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C It occurs at equilibrium, i.e., when the. At 8 0 ∘ c 80^{\circ }c Water freezes at _____ k. 400 200 760 5 the theoretical zero volume temperature of a gas the. What is the approximate vapor pressure when the gas condenses at 80°c? Through this graph, one can find the approximate. What is the approximate vapor pressure when the. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.slideserve.com
PPT Learning Log PowerPoint Presentation, free download ID3217913 What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C 【solved】click here to get an answer to your question : The _____ vapor pressure at high altitudes causes a liquid to boil at a _____ temperature. At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially greater than the pressure at 80.0°c, as we would expect. Water freezes at _____ k. Vapor pressure is the pressure exerted by. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From webmis.highland.cc.il.us
Vapor Pressure What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C The given image of the graph explains the relation between the pressure and the temperature of the substance. 400 200 760 5 the theoretical zero volume temperature of a gas the. The _____ vapor pressure at high altitudes causes a liquid to boil at a _____ temperature. What is the approximate vapor pressure when the gas condenses at 80 °c?. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C Water freezes at _____ k. Vapor pressure is the pressure exerted by the vapor molecules of a substance in a closed system. 1 the vapor pressure of a liquid is the pressure at which its gas phase is in equilibrium with its liquid phase at a given temperature. At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.chegg.com
Solved In the chart are approximate vapor What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C It occurs at equilibrium, i.e., when the. At 160°c, liquid hg has a vapor pressure of 4.21 torr, substantially greater than the pressure at 80.0°c, as we would expect. Vapor pressure is the pressure exerted by the vapor molecules of a substance in a closed system. You can apply the ideal gas law for every gas at a density low. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.chem.fsu.edu
Properties of Liquids What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C At 8 0 ∘ c 80^{\circ }c 【solved】click here to get an answer to your question : Vapor pressure is the pressure exerted by the vapor molecules of a substance in a closed system. Water freezes at _____ k. The _____ vapor pressure at high altitudes causes a liquid to boil at a _____ temperature. At 160°c, liquid hg has. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.wikiwand.com
Vapor pressure Wikiwand What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C Water freezes at _____ k. 1 the vapor pressure of a liquid is the pressure at which its gas phase is in equilibrium with its liquid phase at a given temperature. It occurs at equilibrium, i.e., when the. The given image of the graph explains the relation between the pressure and the temperature of the substance. What is the approximate. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.
From www.eng-tips.com
LNG vapor pressure Chemical process engineering EngTips What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C You can apply the ideal gas law for every gas at a density low enough to prevent the emergence of strong intermolecular forces. The _____ vapor pressure at high altitudes causes a liquid to boil at a _____ temperature. It occurs at equilibrium, i.e., when the. 400 200 760 5 the theoretical zero volume temperature of a gas the. At. What Is The Approximate Vapor Pressure When The Gas Condenses At 80 C.