Lab Method For Determining Concentration . Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Chemists use many different methods to define concentrations, some of which. Which unit you use depends on. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. There are multiple units of concentration. To determine the amounts or concentrations of substances present in a sample, chemists use a combination of chemical reactions and stoichiometric calculations in a methodology. Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. Calculate the concentration of ions in a soluble ionic compound. What concentration measure changes with temperature?
from studylib.net
Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. To determine the amounts or concentrations of substances present in a sample, chemists use a combination of chemical reactions and stoichiometric calculations in a methodology. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Chemists use many different methods to define concentrations, some of which. Calculate the concentration of ions in a soluble ionic compound. There are multiple units of concentration. What concentration measure changes with temperature? Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Which unit you use depends on.
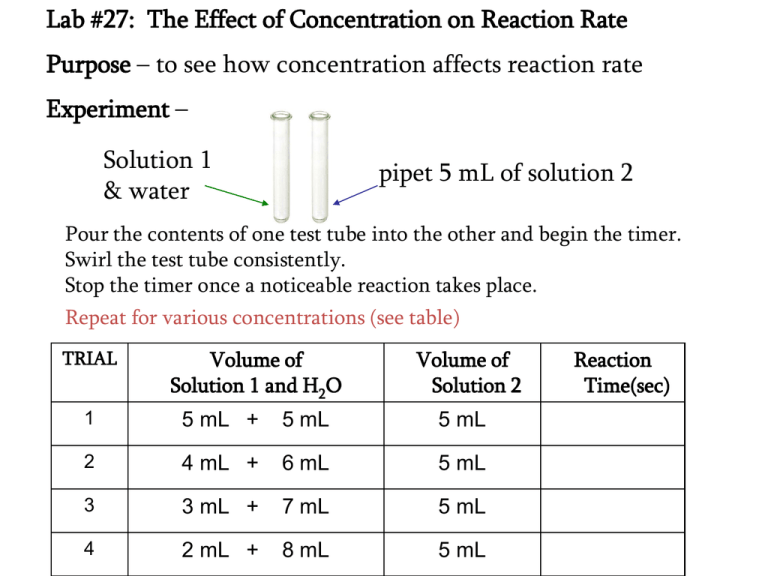
Lab 27 The Effect of Concentration on Reaction Rate Purpose
Lab Method For Determining Concentration Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Which unit you use depends on. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. To determine the amounts or concentrations of substances present in a sample, chemists use a combination of chemical reactions and stoichiometric calculations in a methodology. Chemists use many different methods to define concentrations, some of which. What concentration measure changes with temperature? Calculate the concentration of ions in a soluble ionic compound. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. There are multiple units of concentration.
From chem.libretexts.org
4.5 Concentration of Solutions Chemistry LibreTexts Lab Method For Determining Concentration Calculate the concentration of ions in a soluble ionic compound. Chemists use many different methods to define concentrations, some of which. What concentration measure changes with temperature? Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Outline the steps to make a solution of a desired concentration from a solid. Lab Method For Determining Concentration.
From www.sliderbase.com
Titration Lab Method For Determining Concentration To determine the amounts or concentrations of substances present in a sample, chemists use a combination of chemical reactions and stoichiometric calculations in a methodology. Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Chemists use many different methods to define concentrations, some of which. Outline the steps to make. Lab Method For Determining Concentration.
From www.studocu.com
Exp11 Conductometry new Experiment 11 2019 SAvzianova Conductometry Lab Method For Determining Concentration Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Which unit you use depends on. What concentration measure changes with temperature? Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. Chemists use many different methods to define concentrations, some of which. Concentration. Lab Method For Determining Concentration.
From study.com
How to Determine the Order of Reaction by Comparing Initial Rates of Lab Method For Determining Concentration Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. Which unit you use depends on. Calculate the concentration of ions in a soluble ionic compound. Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Concentration is an expression of how much solute. Lab Method For Determining Concentration.
From extension.uga.edu
Understanding Laboratory Wastewater Tests I. ORGANICS (BOD, COD, TOC Lab Method For Determining Concentration Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Which unit you use depends on. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. To determine. Lab Method For Determining Concentration.
From www.vrogue.co
Acid Base Titration A Concentration Of Acetic Acid In vrogue.co Lab Method For Determining Concentration Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. What concentration measure changes with temperature? Calculate the concentration of ions in a soluble ionic compound. Which unit you use depends on. To determine the amounts or concentrations of substances present in a sample, chemists use a combination of chemical reactions and stoichiometric calculations. Lab Method For Determining Concentration.
From www.youtube.com
IGCSE Chemistry Rates of Reaction YouTube Lab Method For Determining Concentration There are multiple units of concentration. Which unit you use depends on. Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. What concentration measure changes with temperature? Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. Calculate the concentration of ions in a. Lab Method For Determining Concentration.
From www.youtube.com
Generating Standard Curve and Determining Concentration of Unknown Lab Method For Determining Concentration There are multiple units of concentration. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. Calculate the concentration of ions in a soluble ionic compound. Chemists use many different methods to define concentrations, some of which. Concentration is an expression of how much solute is dissolved in a solvent in a chemical. Lab Method For Determining Concentration.
From slidetodoc.com
Titration A laboratory method for determining the concentration Lab Method For Determining Concentration Chemists use many different methods to define concentrations, some of which. To determine the amounts or concentrations of substances present in a sample, chemists use a combination of chemical reactions and stoichiometric calculations in a methodology. Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. Calculate the concentration of ions in a soluble. Lab Method For Determining Concentration.
From www.youtube.com
Estimation of Cu2+ ions by Iodometric Titration Method Iodometric Lab Method For Determining Concentration Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Which unit you use depends on. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. Chemists use many different methods to define concentrations, some of which. What concentration measure changes with temperature? To. Lab Method For Determining Concentration.
From ecampusontario.pressbooks.pub
Chapter 8 Lab Overview and Background Information BBS OER Lab Manual Lab Method For Determining Concentration Chemists use many different methods to define concentrations, some of which. To determine the amounts or concentrations of substances present in a sample, chemists use a combination of chemical reactions and stoichiometric calculations in a methodology. There are multiple units of concentration. What concentration measure changes with temperature? Outline the steps to make a solution of a desired concentration from. Lab Method For Determining Concentration.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Lab Method For Determining Concentration Chemists use many different methods to define concentrations, some of which. What concentration measure changes with temperature? Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Which unit you use depends on. Calculate. Lab Method For Determining Concentration.
From chem.libretexts.org
Chapter 14.4 Using Graphs to Determine Rate Laws, Rate Constants and Lab Method For Determining Concentration Calculate the concentration of ions in a soluble ionic compound. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. Explore the concept of solution concentration, including its various types such as molarity and molality, the importance. Lab Method For Determining Concentration.
From ivypanda.com
Determining Protein Concentration using Kjeldahl Method and Fat Content Lab Method For Determining Concentration Chemists use many different methods to define concentrations, some of which. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. What concentration measure changes with temperature? Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. To determine the amounts or concentrations of substances present. Lab Method For Determining Concentration.
From studylib.net
Determining the Concentration of a Solution Beer`s Law Lab Method For Determining Concentration Calculate the concentration of ions in a soluble ionic compound. There are multiple units of concentration. Which unit you use depends on. Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. To determine the amounts or concentrations of substances present in a sample, chemists use a combination of chemical reactions. Lab Method For Determining Concentration.
From www.chegg.com
Solved Titration for Acetic Acid in Vinegar Lab Report Lab Method For Determining Concentration Calculate the concentration of ions in a soluble ionic compound. To determine the amounts or concentrations of substances present in a sample, chemists use a combination of chemical reactions and stoichiometric calculations in a methodology. Chemists use many different methods to define concentrations, some of which. Concentration is an expression of how much solute is dissolved in a solvent in. Lab Method For Determining Concentration.
From microbeonline.com
Broth Dilution Method for MIC Determination • Microbe Online Lab Method For Determining Concentration Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. What concentration measure changes with temperature? To determine. Lab Method For Determining Concentration.
From www.researchgate.net
Flow chart of the concentration method of water samples Download Lab Method For Determining Concentration Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. Calculate the concentration of ions in a soluble ionic compound. There are multiple units of concentration. To determine the amounts or concentrations of substances present in a. Lab Method For Determining Concentration.
From microbeonline.com
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Lab Method For Determining Concentration What concentration measure changes with temperature? There are multiple units of concentration. Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Chemists use many different methods to define concentrations, some of which. Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. To determine. Lab Method For Determining Concentration.
From www.knowledgeuniverseonline.com
Most Online Prospecting Physics Chemistry Download List of Practicals Lab Method For Determining Concentration Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Which unit you use depends on. There are multiple units of concentration. To determine the amounts or concentrations of substances present in a sample, chemists use a combination of chemical reactions and stoichiometric calculations in a methodology. What concentration measure changes with temperature?. Lab Method For Determining Concentration.
From www.studocu.com
Experiment 1 Solution preparation Concentrations of Solutions The Lab Method For Determining Concentration Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. Which unit you use depends on. Chemists use many different methods to define concentrations, some of which. Calculate the concentration of ions in a. Lab Method For Determining Concentration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Lab Method For Determining Concentration Chemists use many different methods to define concentrations, some of which. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. What concentration measure changes with temperature? Knowing the concentration of solutes is important in controlling. Lab Method For Determining Concentration.
From www.numerade.com
Titration for Acetic Acid in Vinegar Lab Report Exercise 1 Lab Method For Determining Concentration Calculate the concentration of ions in a soluble ionic compound. Which unit you use depends on. What concentration measure changes with temperature? Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. There are multiple units of concentration. Outline the steps to make a solution of a desired concentration from a. Lab Method For Determining Concentration.
From bio.libretexts.org
11.8 Testing the Effectiveness of Antimicrobial Chemicals and Drugs Lab Method For Determining Concentration Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. There are multiple units of concentration. What concentration measure changes with temperature? Calculate the concentration of ions in a soluble ionic compound. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. Which unit. Lab Method For Determining Concentration.
From studylib.net
Lab 27 The Effect of Concentration on Reaction Rate Purpose Lab Method For Determining Concentration Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. What concentration measure changes with temperature? Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Calculate. Lab Method For Determining Concentration.
From www.youtube.com
Calculate Concentration (Example) YouTube Lab Method For Determining Concentration To determine the amounts or concentrations of substances present in a sample, chemists use a combination of chemical reactions and stoichiometric calculations in a methodology. Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions.. Lab Method For Determining Concentration.
From www.mdpi.com
MPs Free FullText Simple Acid Digestion Procedure for the Lab Method For Determining Concentration Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. What concentration measure changes with temperature? Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. Calculate the concentration of ions in a soluble ionic compound. To determine the amounts or concentrations of substances present. Lab Method For Determining Concentration.
From slidetodoc.com
Titration and AcidBase Neutralization Titration A laboratory method Lab Method For Determining Concentration Calculate the concentration of ions in a soluble ionic compound. Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Which unit you use depends on. Chemists use many different methods to define concentrations, some of which.. Lab Method For Determining Concentration.
From www.chegg.com
Solved CHEM 1211 Lab Manual Revised 05/2018 Determining Lab Method For Determining Concentration What concentration measure changes with temperature? There are multiple units of concentration. To determine the amounts or concentrations of substances present in a sample, chemists use a combination of chemical reactions and stoichiometric calculations in a methodology. Calculate the concentration of ions in a soluble ionic compound. Outline the steps to make a solution of a desired concentration from a. Lab Method For Determining Concentration.
From www.slideserve.com
PPT ENZYME CATALYSIS LAB PowerPoint Presentation, free download ID Lab Method For Determining Concentration What concentration measure changes with temperature? Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Calculate the concentration of ions in a soluble ionic compound. There are multiple units of concentration. Chemists use many. Lab Method For Determining Concentration.
From microbe-investigations.com
Minimum Inhibitory Concentration (MIC) Test Lab Method For Determining Concentration There are multiple units of concentration. Which unit you use depends on. What concentration measure changes with temperature? Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. Outline the steps to make a solution. Lab Method For Determining Concentration.
From studylib.net
Standardizing a Sodium Hydroxide (NaOH) Solution Lab Method For Determining Concentration There are multiple units of concentration. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Calculate the concentration of ions in a soluble ionic compound. What concentration measure changes with temperature? Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Chemists use. Lab Method For Determining Concentration.
From www.chegg.com
Solved 4. Effect of substrate concentration. The following Lab Method For Determining Concentration There are multiple units of concentration. Calculate the concentration of ions in a soluble ionic compound. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Explore the concept of solution concentration, including its various types such as molarity and molality, the importance of accurate. Chemists use many different methods to define concentrations,. Lab Method For Determining Concentration.
From studylib.net
Enzyme Activity ThinkChemistry Lab Method For Determining Concentration To determine the amounts or concentrations of substances present in a sample, chemists use a combination of chemical reactions and stoichiometric calculations in a methodology. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Which unit you use depends on. Outline the steps to make a solution of a desired concentration from. Lab Method For Determining Concentration.
From chem.libretexts.org
4.5 Concentration of Solutions Chemistry LibreTexts Lab Method For Determining Concentration Calculate the concentration of ions in a soluble ionic compound. What concentration measure changes with temperature? Which unit you use depends on. To determine the amounts or concentrations of substances present in a sample, chemists use a combination of chemical reactions and stoichiometric calculations in a methodology. Explore the concept of solution concentration, including its various types such as molarity. Lab Method For Determining Concentration.