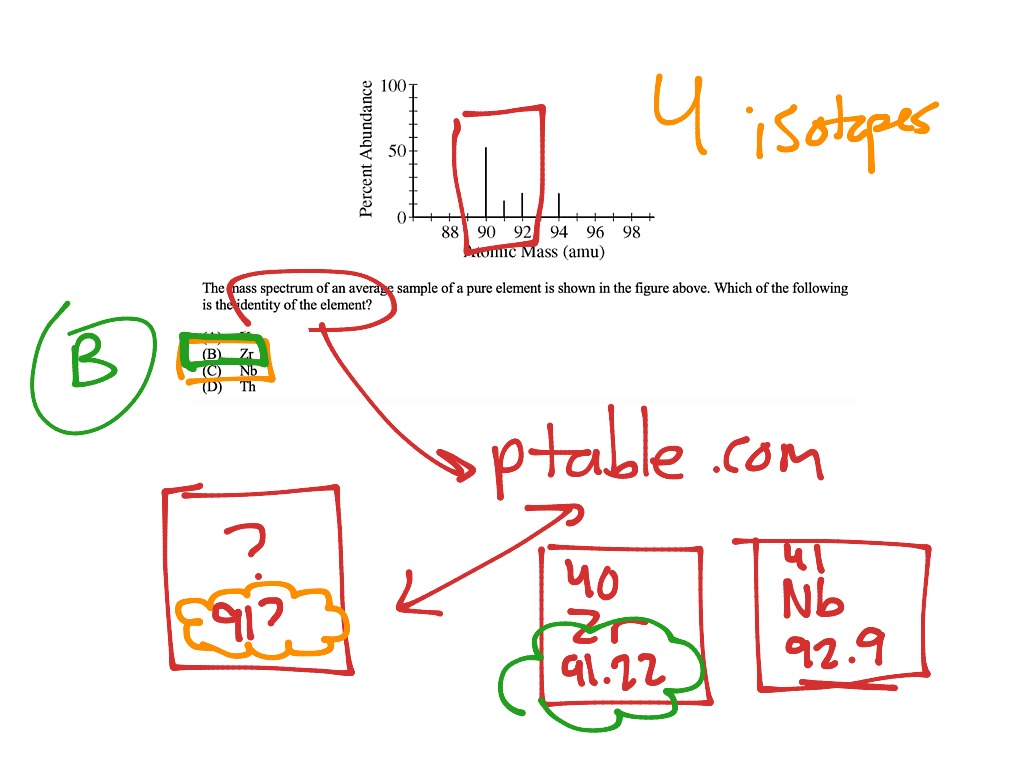
Mass Spectrometry Ap Chemistry . (a) the mass spectrum of a pure sample of si is shown below. In the analytical technique of mass spectrometry, atoms or molecules are ionized. A mass spectrometer provides this information in the form of a. Mass spectroscopy is the principle technique used to study isotopes. Includes full solutions and score reporting. 🔬 scientists analyze unknown substances to identify elements. It is used to both count and weigh atoms in a sample, just not in the. The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer. Answer the following questions about the element si and some of its compounds. (i) how many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of si ? 📊 mass spectroscopy reveals atomic masses and isotopes.
from www.showme.com
(a) the mass spectrum of a pure sample of si is shown below. A mass spectrometer provides this information in the form of a. Mass spectroscopy is the principle technique used to study isotopes. (i) how many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of si ? Answer the following questions about the element si and some of its compounds. In the analytical technique of mass spectrometry, atoms or molecules are ionized. 📊 mass spectroscopy reveals atomic masses and isotopes. 🔬 scientists analyze unknown substances to identify elements. Includes full solutions and score reporting. It is used to both count and weigh atoms in a sample, just not in the.
Mass Spectroscopy of Elements Science, mass spectrometry, AP
Mass Spectrometry Ap Chemistry 🔬 scientists analyze unknown substances to identify elements. Answer the following questions about the element si and some of its compounds. (i) how many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of si ? (a) the mass spectrum of a pure sample of si is shown below. It is used to both count and weigh atoms in a sample, just not in the. 🔬 scientists analyze unknown substances to identify elements. Mass spectroscopy is the principle technique used to study isotopes. Includes full solutions and score reporting. The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer. 📊 mass spectroscopy reveals atomic masses and isotopes. In the analytical technique of mass spectrometry, atoms or molecules are ionized. A mass spectrometer provides this information in the form of a.
From www.slideserve.com
PPT Introduction to Mass Spectrometry (MS) PowerPoint Presentation Mass Spectrometry Ap Chemistry A mass spectrometer provides this information in the form of a. It is used to both count and weigh atoms in a sample, just not in the. (i) how many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of si ? Includes full solutions and score reporting. In the analytical technique. Mass Spectrometry Ap Chemistry.
From chem.libretexts.org
6.4 Isotope Abundance Chemistry LibreTexts Mass Spectrometry Ap Chemistry A mass spectrometer provides this information in the form of a. (a) the mass spectrum of a pure sample of si is shown below. Answer the following questions about the element si and some of its compounds. (i) how many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of si ?. Mass Spectrometry Ap Chemistry.
From www.youtube.com
Mass Spectrometry (AP) YouTube Mass Spectrometry Ap Chemistry The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer. (i) how many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of si ? 📊 mass spectroscopy reveals atomic masses and isotopes. A mass spectrometer provides this information in the. Mass Spectrometry Ap Chemistry.
From www.youtube.com
Mass Spectrometry AP Chemistry Sample Problems YouTube Mass Spectrometry Ap Chemistry (a) the mass spectrum of a pure sample of si is shown below. A mass spectrometer provides this information in the form of a. It is used to both count and weigh atoms in a sample, just not in the. Answer the following questions about the element si and some of its compounds. The mass and relative abundance of each. Mass Spectrometry Ap Chemistry.
From www.slideserve.com
PPT AP Chemistry Big Idea 1 MASS SPECTROMETRY PowerPoint Presentation Mass Spectrometry Ap Chemistry In the analytical technique of mass spectrometry, atoms or molecules are ionized. (a) the mass spectrum of a pure sample of si is shown below. 🔬 scientists analyze unknown substances to identify elements. The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer. It is used to both count. Mass Spectrometry Ap Chemistry.
From mungfali.com
Mass Spectrometry Principle Mass Spectrometry Ap Chemistry Answer the following questions about the element si and some of its compounds. (i) how many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of si ? 📊 mass spectroscopy reveals atomic masses and isotopes. It is used to both count and weigh atoms in a sample, just not in the.. Mass Spectrometry Ap Chemistry.
From www.reddit.com
[AP Chemistry] How to read a mass spectroscopy? r/HomeworkHelp Mass Spectrometry Ap Chemistry (a) the mass spectrum of a pure sample of si is shown below. Answer the following questions about the element si and some of its compounds. (i) how many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of si ? A mass spectrometer provides this information in the form of a.. Mass Spectrometry Ap Chemistry.
From lessonmagicholtzmann.z19.web.core.windows.net
Mass Spectrometry Worksheet With Answers Mass Spectrometry Ap Chemistry (i) how many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of si ? In the analytical technique of mass spectrometry, atoms or molecules are ionized. Answer the following questions about the element si and some of its compounds. (a) the mass spectrum of a pure sample of si is shown. Mass Spectrometry Ap Chemistry.
From brainly.com
The mass spectrum of a sample of a pure element is shown above. Based Mass Spectrometry Ap Chemistry 🔬 scientists analyze unknown substances to identify elements. (a) the mass spectrum of a pure sample of si is shown below. Includes full solutions and score reporting. The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer. Answer the following questions about the element si and some of its. Mass Spectrometry Ap Chemistry.
From www.youtube.com
Mass spectrometry Atomic structure and properties AP Chemistry Mass Spectrometry Ap Chemistry Mass spectroscopy is the principle technique used to study isotopes. 🔬 scientists analyze unknown substances to identify elements. A mass spectrometer provides this information in the form of a. Answer the following questions about the element si and some of its compounds. Includes full solutions and score reporting. 📊 mass spectroscopy reveals atomic masses and isotopes. It is used to. Mass Spectrometry Ap Chemistry.
From thechemistrynotes.com
Mass Spectrometry Mass Spectrometry Ap Chemistry The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer. (a) the mass spectrum of a pure sample of si is shown below. 🔬 scientists analyze unknown substances to identify elements. A mass spectrometer provides this information in the form of a. Mass spectroscopy is the principle technique used. Mass Spectrometry Ap Chemistry.
From www.chegg.com
Solved Exp.2B Worksheet Org. Chem. I Lab Mass spectrometry Mass Spectrometry Ap Chemistry 📊 mass spectroscopy reveals atomic masses and isotopes. In the analytical technique of mass spectrometry, atoms or molecules are ionized. (a) the mass spectrum of a pure sample of si is shown below. Includes full solutions and score reporting. Answer the following questions about the element si and some of its compounds. The mass and relative abundance of each isotope. Mass Spectrometry Ap Chemistry.
From slideplayer.com
Spectroscopy for AP Chemistry ppt download Mass Spectrometry Ap Chemistry Includes full solutions and score reporting. A mass spectrometer provides this information in the form of a. The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer. In the analytical technique of mass spectrometry, atoms or molecules are ionized. It is used to both count and weigh atoms in. Mass Spectrometry Ap Chemistry.
From www.savemyexams.co.uk
Mass spectrometry (3.6.2) AQA A Level Chemistry Revision Notes 2017 Mass Spectrometry Ap Chemistry It is used to both count and weigh atoms in a sample, just not in the. (i) how many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of si ? (a) the mass spectrum of a pure sample of si is shown below. In the analytical technique of mass spectrometry, atoms. Mass Spectrometry Ap Chemistry.
From www.instantuition.com
Mass Spectrometry of Chlorine O Level Chemistry Mass Spectrometry Ap Chemistry Includes full solutions and score reporting. 📊 mass spectroscopy reveals atomic masses and isotopes. The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer. 🔬 scientists analyze unknown substances to identify elements. Answer the following questions about the element si and some of its compounds. A mass spectrometer provides. Mass Spectrometry Ap Chemistry.
From www.studocu.com
AP chem Isotopes and Mass Spectrometry A PSI AP Chemistry Activity Mass Spectrometry Ap Chemistry (i) how many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of si ? Answer the following questions about the element si and some of its compounds. (a) the mass spectrum of a pure sample of si is shown below. A mass spectrometer provides this information in the form of a.. Mass Spectrometry Ap Chemistry.
From www.youtube.com
Mass Spectrometry YouTube Mass Spectrometry Ap Chemistry It is used to both count and weigh atoms in a sample, just not in the. 📊 mass spectroscopy reveals atomic masses and isotopes. (i) how many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of si ? (a) the mass spectrum of a pure sample of si is shown below.. Mass Spectrometry Ap Chemistry.
From www.pinterest.com
Khan Academy Mass spectrometry, Spectrometers, Physics and mathematics Mass Spectrometry Ap Chemistry (i) how many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of si ? A mass spectrometer provides this information in the form of a. (a) the mass spectrum of a pure sample of si is shown below. 📊 mass spectroscopy reveals atomic masses and isotopes. Includes full solutions and score. Mass Spectrometry Ap Chemistry.
From www.showme.com
Mass Spectroscopy of Elements Science, mass spectrometry, AP Mass Spectrometry Ap Chemistry 📊 mass spectroscopy reveals atomic masses and isotopes. Includes full solutions and score reporting. Mass spectroscopy is the principle technique used to study isotopes. (a) the mass spectrum of a pure sample of si is shown below. Answer the following questions about the element si and some of its compounds. 🔬 scientists analyze unknown substances to identify elements. In the. Mass Spectrometry Ap Chemistry.
From study.com
How to Identify an Element from Its Mass Spectrum Chemistry Mass Spectrometry Ap Chemistry Mass spectroscopy is the principle technique used to study isotopes. Includes full solutions and score reporting. (a) the mass spectrum of a pure sample of si is shown below. 🔬 scientists analyze unknown substances to identify elements. It is used to both count and weigh atoms in a sample, just not in the. (i) how many protons and how many. Mass Spectrometry Ap Chemistry.
From www.youtube.com
1.2. Mass spectroscopy of Elements College Board AP Chemistry YouTube Mass Spectrometry Ap Chemistry Answer the following questions about the element si and some of its compounds. Mass spectroscopy is the principle technique used to study isotopes. 🔬 scientists analyze unknown substances to identify elements. Includes full solutions and score reporting. The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer. 📊 mass. Mass Spectrometry Ap Chemistry.
From www.youtube.com
Mass Spectrometry EDx Learning HSC Biology YouTube Mass Spectrometry Ap Chemistry The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer. (a) the mass spectrum of a pure sample of si is shown below. 📊 mass spectroscopy reveals atomic masses and isotopes. Answer the following questions about the element si and some of its compounds. Includes full solutions and score. Mass Spectrometry Ap Chemistry.
From www.slideserve.com
PPT AP Chemistry Big Idea 1 MASS SPECTROMETRY PowerPoint Presentation Mass Spectrometry Ap Chemistry In the analytical technique of mass spectrometry, atoms or molecules are ionized. Includes full solutions and score reporting. A mass spectrometer provides this information in the form of a. It is used to both count and weigh atoms in a sample, just not in the. (i) how many protons and how many neutrons are in the nucleus of an atom. Mass Spectrometry Ap Chemistry.
From in.pinterest.com
NMR spectroscopy An Easy Introduction Chemistry Steps Chemistry Mass Spectrometry Ap Chemistry Answer the following questions about the element si and some of its compounds. 🔬 scientists analyze unknown substances to identify elements. The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer. (a) the mass spectrum of a pure sample of si is shown below. It is used to both. Mass Spectrometry Ap Chemistry.
From www.youtube.com
Mass Spectrometry in Organic Chemistry // HSC Chemistry YouTube Mass Spectrometry Ap Chemistry Mass spectroscopy is the principle technique used to study isotopes. 🔬 scientists analyze unknown substances to identify elements. The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer. 📊 mass spectroscopy reveals atomic masses and isotopes. It is used to both count and weigh atoms in a sample, just. Mass Spectrometry Ap Chemistry.
From www.youtube.com
New AP Chemistry Material Mass Spectrometry YouTube Mass Spectrometry Ap Chemistry 📊 mass spectroscopy reveals atomic masses and isotopes. A mass spectrometer provides this information in the form of a. 🔬 scientists analyze unknown substances to identify elements. Mass spectroscopy is the principle technique used to study isotopes. Answer the following questions about the element si and some of its compounds. The mass and relative abundance of each isotope of an. Mass Spectrometry Ap Chemistry.
From www.pinterest.com
Mass spectrometry simple diagram Mass spectrometry, Medical Mass Spectrometry Ap Chemistry It is used to both count and weigh atoms in a sample, just not in the. Answer the following questions about the element si and some of its compounds. In the analytical technique of mass spectrometry, atoms or molecules are ionized. 📊 mass spectroscopy reveals atomic masses and isotopes. Includes full solutions and score reporting. 🔬 scientists analyze unknown substances. Mass Spectrometry Ap Chemistry.
From studylib.net
HW 83 Photoelectron Spectroscopy WKS Key Mass Spectrometry Ap Chemistry 📊 mass spectroscopy reveals atomic masses and isotopes. Includes full solutions and score reporting. 🔬 scientists analyze unknown substances to identify elements. (a) the mass spectrum of a pure sample of si is shown below. In the analytical technique of mass spectrometry, atoms or molecules are ionized. (i) how many protons and how many neutrons are in the nucleus of. Mass Spectrometry Ap Chemistry.
From www.slideserve.com
PPT AP Chemistry Big Idea 1 MASS SPECTROMETRY PowerPoint Presentation Mass Spectrometry Ap Chemistry Includes full solutions and score reporting. 🔬 scientists analyze unknown substances to identify elements. The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer. In the analytical technique of mass spectrometry, atoms or molecules are ionized. Mass spectroscopy is the principle technique used to study isotopes. 📊 mass spectroscopy. Mass Spectrometry Ap Chemistry.
From www.chemistrystudent.com
Mass Spectrometry (ALevel) ChemistryStudent Mass Spectrometry Ap Chemistry (a) the mass spectrum of a pure sample of si is shown below. In the analytical technique of mass spectrometry, atoms or molecules are ionized. 📊 mass spectroscopy reveals atomic masses and isotopes. It is used to both count and weigh atoms in a sample, just not in the. Answer the following questions about the element si and some of. Mass Spectrometry Ap Chemistry.
From www.youtube.com
Topic 1.2 Mass Spectroscopy of Elements YouTube Mass Spectrometry Ap Chemistry (i) how many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of si ? It is used to both count and weigh atoms in a sample, just not in the. The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer.. Mass Spectrometry Ap Chemistry.
From www.coursehero.com
[Solved] Mass Spectroscopy worksheet mass spectroscopy worksheet The Mass Spectrometry Ap Chemistry Answer the following questions about the element si and some of its compounds. (a) the mass spectrum of a pure sample of si is shown below. 📊 mass spectroscopy reveals atomic masses and isotopes. Mass spectroscopy is the principle technique used to study isotopes. Includes full solutions and score reporting. In the analytical technique of mass spectrometry, atoms or molecules. Mass Spectrometry Ap Chemistry.
From www.scribd.com
Mass Spectroscopy Worksheet PDF Mass Spectrometry Ap Chemistry In the analytical technique of mass spectrometry, atoms or molecules are ionized. 📊 mass spectroscopy reveals atomic masses and isotopes. The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer. Answer the following questions about the element si and some of its compounds. Mass spectroscopy is the principle technique. Mass Spectrometry Ap Chemistry.
From www.adriandingleschemistrypages.com
Developing more thoughts on Mass Spec Adrian Dingle's Chemistry Pages Mass Spectrometry Ap Chemistry Answer the following questions about the element si and some of its compounds. (a) the mass spectrum of a pure sample of si is shown below. The mass and relative abundance of each isotope of an element in a sample can be determined using a mass spectrometer. 📊 mass spectroscopy reveals atomic masses and isotopes. It is used to both. Mass Spectrometry Ap Chemistry.
From schoolbag.info
THE PERIODIC TABLE Atoms, Elements, and the Building Blocks of Matter Mass Spectrometry Ap Chemistry Includes full solutions and score reporting. (a) the mass spectrum of a pure sample of si is shown below. A mass spectrometer provides this information in the form of a. (i) how many protons and how many neutrons are in the nucleus of an atom of the most abundant isotope of si ? Mass spectroscopy is the principle technique used. Mass Spectrometry Ap Chemistry.