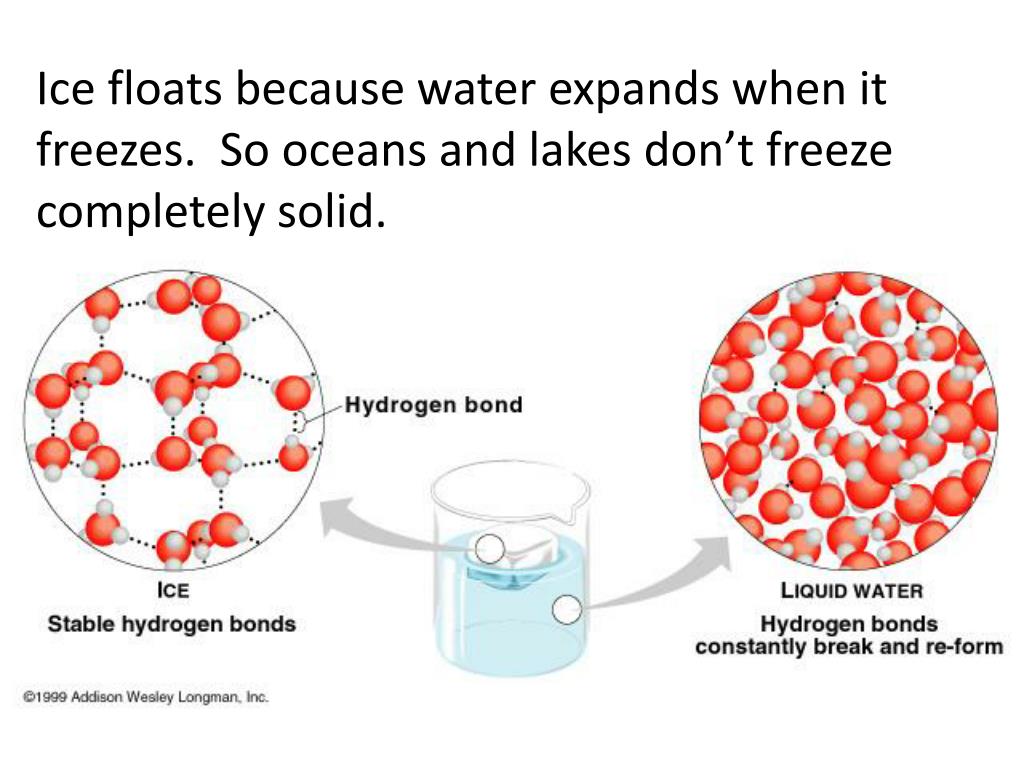
Why Does Ice Float On Water Hydrogen Bonds . So, the buoyant force will balance out the force of. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. The water molecules in ice take up about 9% more The hydrogen bonds in ice are more. So, why does ice float on water? This is almost exactly the same as the expression for f b, with one difference:
from thefactbase.com
The hydrogen bonds in ice are more. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. So, the buoyant force will balance out the force of. When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. So, why does ice float on water? The water molecules in ice take up about 9% more This is almost exactly the same as the expression for f b, with one difference: The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms.
Water expands when it freezes ice has a lesser density than water so an
Why Does Ice Float On Water Hydrogen Bonds The water molecules in ice take up about 9% more The hydrogen bonds in ice are more. So, why does ice float on water? So, the buoyant force will balance out the force of. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. This is almost exactly the same as the expression for f b, with one difference: The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. The water molecules in ice take up about 9% more When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice.
From www.youtube.com
Why does ice float in water? Zaidan and Charles Morton YouTube Why Does Ice Float On Water Hydrogen Bonds This is almost exactly the same as the expression for f b, with one difference: The water molecules in ice take up about 9% more The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. So, the buoyant force will balance out the force of. So, why does ice float on water?. Why Does Ice Float On Water Hydrogen Bonds.
From mungfali.com
Ice Hydrogen Bonding Why Does Ice Float On Water Hydrogen Bonds So, the buoyant force will balance out the force of. The hydrogen bonds in ice are more. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. This is almost exactly the same as the expression for f b, with one difference: So, why does ice float on water?. Why Does Ice Float On Water Hydrogen Bonds.
From www.numerade.com
SOLVED 12. Hydrogen Bonding Hydrogen bonds form between adjacent water Why Does Ice Float On Water Hydrogen Bonds Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. So, why does ice float on water? The hydrogen bonds in ice are more. The water. Why Does Ice Float On Water Hydrogen Bonds.
From answerdbmaligner.z21.web.core.windows.net
How Does Ice Float Why Does Ice Float On Water Hydrogen Bonds The hydrogen bonds in ice are more. So, why does ice float on water? When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. The water molecules in ice take up about 9% more This is almost exactly the same as the expression for f b, with. Why Does Ice Float On Water Hydrogen Bonds.
From www.numerade.com
SOLVED 12. Hydrogen Bonding Hydrogen bonds form between adjacent water Why Does Ice Float On Water Hydrogen Bonds The hydrogen bonds in ice are more. So, why does ice float on water? Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. So, the. Why Does Ice Float On Water Hydrogen Bonds.
From slidesharenow.blogspot.com
Why Does Ice Float On Water slideshare Why Does Ice Float On Water Hydrogen Bonds When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. The hydrogen bonds in ice are more. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. This is almost exactly the same as the expression. Why Does Ice Float On Water Hydrogen Bonds.
From www.slideserve.com
PPT Ch 1 The Chemistry of Life PowerPoint Presentation, free Why Does Ice Float On Water Hydrogen Bonds This is almost exactly the same as the expression for f b, with one difference: The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. So, why does ice float on water? When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into. Why Does Ice Float On Water Hydrogen Bonds.
From animalia-life.club
Hydrogen Bonds Ice Why Does Ice Float On Water Hydrogen Bonds When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. The water molecules in ice take up about 9% more So, why does ice float on water? Although ice is a solid form of water, ice is lighter than water because the ice is less dense than. Why Does Ice Float On Water Hydrogen Bonds.
From sayngon.com
Are Hydrogen Bonds The Strongest Interactions Between Molecules? Why Does Ice Float On Water Hydrogen Bonds The water molecules in ice take up about 9% more The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. So, the buoyant force will balance out the force of. The hydrogen bonds in ice are more. This is almost exactly the same as the expression for f b, with one difference:. Why Does Ice Float On Water Hydrogen Bonds.
From www.proprofs.com
Water Chapter 3 Flashcards by ProProfs Why Does Ice Float On Water Hydrogen Bonds The hydrogen bonds in ice are more. The water molecules in ice take up about 9% more When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. So, why does ice float on water? The molecules in ice are held further apart by the hydrogen bonds between. Why Does Ice Float On Water Hydrogen Bonds.
From exoqidach.blob.core.windows.net
What Why Does Ice Float On Water at Floyd McKinney blog Why Does Ice Float On Water Hydrogen Bonds Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and. Why Does Ice Float On Water Hydrogen Bonds.
From www.sciencefacts.net
Water Expansion When Freezing Science Facts Why Does Ice Float On Water Hydrogen Bonds The water molecules in ice take up about 9% more So, why does ice float on water? So, the buoyant force will balance out the force of. The hydrogen bonds in ice are more. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. When water freezes into ice, the h 2. Why Does Ice Float On Water Hydrogen Bonds.
From sayngon.com
Are Most Liquids Denser Than Solids? Exploring Density Differences Why Does Ice Float On Water Hydrogen Bonds This is almost exactly the same as the expression for f b, with one difference: The hydrogen bonds in ice are more. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. So, the buoyant force will balance out the force of. When water freezes into ice, the h. Why Does Ice Float On Water Hydrogen Bonds.
From thefactbase.com
Water expands when it freezes ice has a lesser density than water so an Why Does Ice Float On Water Hydrogen Bonds This is almost exactly the same as the expression for f b, with one difference: The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. So, why does ice float on water? The water molecules in ice take up about 9% more When water freezes into ice, the h 2 o molecules. Why Does Ice Float On Water Hydrogen Bonds.
From www.scienceabc.com
Why Does Ice Float On Water? » ScienceABC Why Does Ice Float On Water Hydrogen Bonds When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. The water molecules in ice take up about 9% more Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. The molecules in ice are held. Why Does Ice Float On Water Hydrogen Bonds.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, Why Does Ice Float On Water Hydrogen Bonds The hydrogen bonds in ice are more. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. Although ice is a solid form of water, ice is lighter than. Why Does Ice Float On Water Hydrogen Bonds.
From www.youtube.com
Why does ice float on water? YouTube Why Does Ice Float On Water Hydrogen Bonds The water molecules in ice take up about 9% more Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. So, the buoyant force will balance out the force of. This is almost exactly the same as the expression for f b, with one difference: The molecules in ice. Why Does Ice Float On Water Hydrogen Bonds.
From www.slideserve.com
PPT Chemical and Physical Features of Seawater and the World Ocean Why Does Ice Float On Water Hydrogen Bonds When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. The water molecules in ice take up about 9% more This is almost exactly the same as the expression. Why Does Ice Float On Water Hydrogen Bonds.
From www.reddit.com
ELI5 Why does ice float in water? Isn't ice 'denser' than liquid water Why Does Ice Float On Water Hydrogen Bonds So, the buoyant force will balance out the force of. When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. This is almost exactly the same as the expression for f b, with one difference: The hydrogen bonds in ice are more. Although ice is a solid. Why Does Ice Float On Water Hydrogen Bonds.
From nl.pinterest.com
Why Is Water a Polar Molecule? Hydrogen Atom, Hydrogen Bond, Ionic Why Does Ice Float On Water Hydrogen Bonds The hydrogen bonds in ice are more. When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. So, the buoyant force will balance out the force. Why Does Ice Float On Water Hydrogen Bonds.
From www.dreamstime.com
Why Does Ice Float on Water Infographic Diagram Stock Vector Why Does Ice Float On Water Hydrogen Bonds So, the buoyant force will balance out the force of. So, why does ice float on water? The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. When water freezes into. Why Does Ice Float On Water Hydrogen Bonds.
From slideplayer.com
Water Unit. ppt download Why Does Ice Float On Water Hydrogen Bonds So, why does ice float on water? The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. When water freezes into ice, the h 2 o molecules form hydrogen bonds between. Why Does Ice Float On Water Hydrogen Bonds.
From www.slideserve.com
PPT Chapter 11 Fluid Statics PowerPoint Presentation, free download Why Does Ice Float On Water Hydrogen Bonds When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. So, why does ice float on water? The water molecules in ice take up about 9% more So, the buoyant force will balance out the force of. The hydrogen bonds in ice are more. The molecules in. Why Does Ice Float On Water Hydrogen Bonds.
From thevigyan.com
Why Does Ice Float in Water? The Vigyan Why Does Ice Float On Water Hydrogen Bonds So, the buoyant force will balance out the force of. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. The hydrogen bonds in ice are more. So, why does ice float on water? The water molecules in ice take up about 9% more When water freezes into ice,. Why Does Ice Float On Water Hydrogen Bonds.
From www.livescience.com
Why does ice float? Live Science Why Does Ice Float On Water Hydrogen Bonds The water molecules in ice take up about 9% more The hydrogen bonds in ice are more. This is almost exactly the same as the expression for f b, with one difference: So, why does ice float on water? So, the buoyant force will balance out the force of. When water freezes into ice, the h 2 o molecules form. Why Does Ice Float On Water Hydrogen Bonds.
From academichelp.net
Why Does Ice Float on Water? The Science Behind It Explained Why Does Ice Float On Water Hydrogen Bonds The water molecules in ice take up about 9% more So, why does ice float on water? The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. This is almost exactly the same as the expression for f b, with one difference: The hydrogen bonds in ice are more. Although ice is. Why Does Ice Float On Water Hydrogen Bonds.
From quizastrictive.z4.web.core.windows.net
Why Can Ice Float On Liquid Water Why Does Ice Float On Water Hydrogen Bonds So, why does ice float on water? This is almost exactly the same as the expression for f b, with one difference: Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen. Why Does Ice Float On Water Hydrogen Bonds.
From animalia-life.club
Hydrogen Bonds Ice Why Does Ice Float On Water Hydrogen Bonds The hydrogen bonds in ice are more. This is almost exactly the same as the expression for f b, with one difference: So, why does ice float on water? When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. The water molecules in ice take up about. Why Does Ice Float On Water Hydrogen Bonds.
From higheducations.com
Why Does Ice Float in Liquid Water? Why Does Ice Float On Water Hydrogen Bonds When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange themselves into an open lattice. The water molecules in ice take up about 9% more The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. So, why does ice float on water? Although ice. Why Does Ice Float On Water Hydrogen Bonds.
From animalia-life.club
Hydrogen Bonds Ice Why Does Ice Float On Water Hydrogen Bonds This is almost exactly the same as the expression for f b, with one difference: So, the buoyant force will balance out the force of. So, why does ice float on water? The water molecules in ice take up about 9% more Although ice is a solid form of water, ice is lighter than water because the ice is less. Why Does Ice Float On Water Hydrogen Bonds.
From slideplayer.com
WATER. ppt download Why Does Ice Float On Water Hydrogen Bonds The hydrogen bonds in ice are more. So, why does ice float on water? So, the buoyant force will balance out the force of. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. When water freezes into ice, the h 2 o molecules form hydrogen bonds between each other and arrange. Why Does Ice Float On Water Hydrogen Bonds.
From techiescientist.com
Why Does Ice Float on Water? Techiescientist Why Does Ice Float On Water Hydrogen Bonds Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. So, the buoyant force will balance out the force of. The water molecules in ice take up about 9% more So,. Why Does Ice Float On Water Hydrogen Bonds.
From slidesharenow.blogspot.com
Why Does Ice Float On Water slideshare Why Does Ice Float On Water Hydrogen Bonds The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. The hydrogen bonds in ice are more. The water molecules in ice take up about 9% more Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. This is almost exactly. Why Does Ice Float On Water Hydrogen Bonds.
From exoxmnlvb.blob.core.windows.net
Why Does My Ice Float at Cynthia Pack blog Why Does Ice Float On Water Hydrogen Bonds The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. So, why does ice float on water? The water molecules in ice take up about 9% more Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. This is almost exactly. Why Does Ice Float On Water Hydrogen Bonds.
From tinyhousegarage.com
Why Does Ice Float On Water Chemistry? Why Does Ice Float On Water Hydrogen Bonds So, the buoyant force will balance out the force of. This is almost exactly the same as the expression for f b, with one difference: The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. The hydrogen bonds in ice are more. Although ice is a solid form of water, ice is. Why Does Ice Float On Water Hydrogen Bonds.