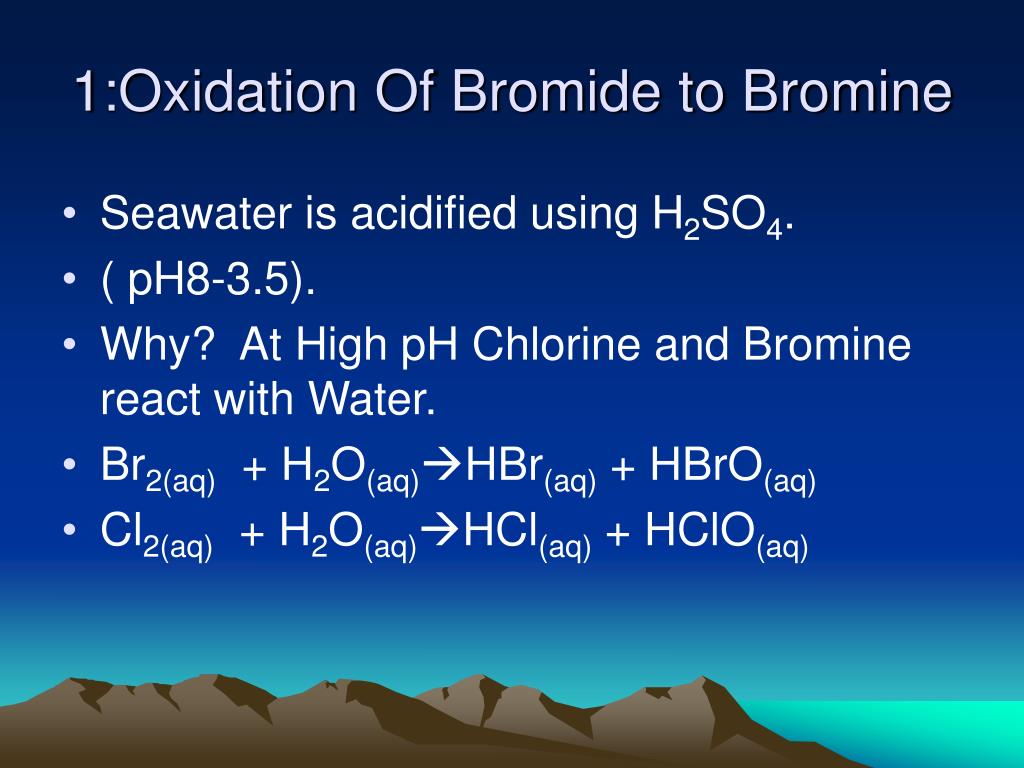
Bromine Water Reducing Agent . The reducing agent undergoes oxidation with the loss of electrons. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Bromide reduces sulfuric acid to sulfur dioxide. The reducing reagent in this reaction is ca. Identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing agents in redox reactions. In the process, bromide ions are oxidized to bromine. The reducing agent is the species that is being oxidized (losing electrons). Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. The brown colour of bromine water turns colourless because bromine molecules are reduced to become bromide ions. So, the carbon electrode that is immersed in the solution of the reducing agent becomes the negative cell. A reducing agent is oxidized,.
from www.slideserve.com
Bromide reduces sulfuric acid to sulfur dioxide. Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. In the process, bromide ions are oxidized to bromine. A reducing agent is oxidized,. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing agents in redox reactions. The brown colour of bromine water turns colourless because bromine molecules are reduced to become bromide ions. The reducing agent undergoes oxidation with the loss of electrons. The reducing agent is the species that is being oxidized (losing electrons). The reducing reagent in this reaction is ca.
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation
Bromine Water Reducing Agent Bromide reduces sulfuric acid to sulfur dioxide. The brown colour of bromine water turns colourless because bromine molecules are reduced to become bromide ions. Identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing agents in redox reactions. Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. Bromide reduces sulfuric acid to sulfur dioxide. The reducing agent undergoes oxidation with the loss of electrons. The reducing agent is the species that is being oxidized (losing electrons). The reducing reagent in this reaction is ca. So, the carbon electrode that is immersed in the solution of the reducing agent becomes the negative cell. A reducing agent is oxidized,. In the process, bromide ions are oxidized to bromine. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor.
From www.vedantu.com
Revision Notes Class 11 Chemistry Ch 8 Redox Reactions Bromine Water Reducing Agent The reducing reagent in this reaction is ca. So, the carbon electrode that is immersed in the solution of the reducing agent becomes the negative cell. The brown colour of bromine water turns colourless because bromine molecules are reduced to become bromide ions. A reducing agent is typically in one of its lower possible oxidation states, and is known as. Bromine Water Reducing Agent.
From www.youtube.com
Experiment of Bromine Water Test🔥 Organic Chemistry Imran Sir Bromine Water Reducing Agent Bromide reduces sulfuric acid to sulfur dioxide. The reducing reagent in this reaction is ca. The reducing agent undergoes oxidation with the loss of electrons. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Bromine molecules, which give the bromine water its brown colour, gain the electrons and are. Bromine Water Reducing Agent.
From www.alamy.com
Hydrogen bromide is a colorless gas that is used as a reducing agent Bromine Water Reducing Agent So, the carbon electrode that is immersed in the solution of the reducing agent becomes the negative cell. A reducing agent is oxidized,. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. The reducing agent undergoes oxidation with the loss of electrons. Bromide reduces sulfuric acid to sulfur dioxide.. Bromine Water Reducing Agent.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation Bromine Water Reducing Agent Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. The brown colour of bromine water turns colourless because bromine molecules are reduced to become bromide ions. The reducing agent is the species that is being oxidized (losing electrons). In the process, bromide ions are oxidized to bromine. So,. Bromine Water Reducing Agent.
From www.sciencephoto.com
Bromine water test for alkenes Stock Image C029/0955 Science Bromine Water Reducing Agent The reducing agent undergoes oxidation with the loss of electrons. A reducing agent is oxidized,. The reducing reagent in this reaction is ca. Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. The brown colour of bromine water turns colourless because bromine molecules are reduced to become bromide. Bromine Water Reducing Agent.
From www.sciencephoto.com
Test tube of bromine water Stock Image C029/0952 Science Photo Bromine Water Reducing Agent In the process, bromide ions are oxidized to bromine. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. The brown colour of bromine water turns colourless because bromine molecules are reduced to become bromide ions. A reducing agent is oxidized,. Bromide reduces sulfuric acid to sulfur dioxide. The reducing. Bromine Water Reducing Agent.
From www.numerade.com
Bromine reacts with sodium iodide to yield iodine and sodium bromide Bromine Water Reducing Agent The brown colour of bromine water turns colourless because bromine molecules are reduced to become bromide ions. Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. Bromide reduces sulfuric acid to sulfur dioxide. A reducing agent is typically in one of its lower possible oxidation states, and is. Bromine Water Reducing Agent.
From www.youtube.com
Bromine Water Test for Saturation and Unsaturation II Procedure II Bromine Water Reducing Agent A reducing agent is oxidized,. The reducing agent undergoes oxidation with the loss of electrons. Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. So, the carbon electrode that is immersed in the solution of the reducing agent becomes the negative cell. In the process, bromide ions are. Bromine Water Reducing Agent.
From brainly.in
Reaction between sodium iodide and bromine water Brainly.in Bromine Water Reducing Agent A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. The brown colour of bromine water turns colourless because bromine molecules are reduced to become bromide ions. Identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing agents in redox reactions. The reducing reagent in. Bromine Water Reducing Agent.
From www.youtube.com
Test for Unsaturation Bromine water Test Baeyer's Reagent Test Bromine Water Reducing Agent The reducing agent is the species that is being oxidized (losing electrons). Identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing agents in redox reactions. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. The reducing agent undergoes oxidation with the loss of. Bromine Water Reducing Agent.
From exclusivepoolparts.com
3 LB NuClo Granular Dechlor Chlorine Bromine Reducing Agent Neutral Bromine Water Reducing Agent In the process, bromide ions are oxidized to bromine. The reducing agent is the species that is being oxidized (losing electrons). Identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing agents in redox reactions. The reducing reagent in this reaction is ca. The brown colour of bromine water turns colourless because bromine molecules are. Bromine Water Reducing Agent.
From www.chemistore.co.za
Bromine water, 500ml Bromine Water Reducing Agent The reducing reagent in this reaction is ca. The reducing agent undergoes oxidation with the loss of electrons. Identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing agents in redox reactions. So, the carbon electrode that is immersed in the solution of the reducing agent becomes the negative cell. The brown colour of bromine. Bromine Water Reducing Agent.
From dir.indiamart.com
Bromine Water Bromide Bromate Solution Latest Price, Manufacturers Bromine Water Reducing Agent A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. The brown colour of bromine water turns colourless because bromine molecules are reduced to become bromide ions. The reducing agent undergoes oxidation with the loss of electrons. A reducing agent is oxidized,. So, the carbon electrode that is immersed in. Bromine Water Reducing Agent.
From www.indiamart.com
500ml Indochem Bromine Water at Rs 126/bottle Liquid Chemical in Bromine Water Reducing Agent Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. The reducing reagent in this reaction is ca. In the process, bromide ions are oxidized to bromine. So, the. Bromine Water Reducing Agent.
From www.slideshare.net
Oxidation & reduction Bromine Water Reducing Agent The reducing agent undergoes oxidation with the loss of electrons. The reducing reagent in this reaction is ca. Identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing agents in redox reactions. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Bromide reduces sulfuric. Bromine Water Reducing Agent.
From dir.indiamart.com
Bromine Water at Best Price in India Bromine Water Reducing Agent The reducing agent is the species that is being oxidized (losing electrons). So, the carbon electrode that is immersed in the solution of the reducing agent becomes the negative cell. The reducing reagent in this reaction is ca. The reducing agent undergoes oxidation with the loss of electrons. Bromine molecules, which give the bromine water its brown colour, gain the. Bromine Water Reducing Agent.
From www.mdpi.com
Membranes Free FullText Roles of Sulfites in Reverse Osmosis (RO Bromine Water Reducing Agent The brown colour of bromine water turns colourless because bromine molecules are reduced to become bromide ions. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Bromide reduces sulfuric acid to sulfur dioxide. So, the carbon electrode that is immersed in the solution of the reducing agent becomes the. Bromine Water Reducing Agent.
From www.youtube.com
Bromine Water Test For Unsaturation Red Brown Solution Practical Bromine Water Reducing Agent Identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing agents in redox reactions. Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. A reducing agent is oxidized,. The brown colour of bromine water turns colourless because bromine molecules are reduced to become. Bromine Water Reducing Agent.
From chiangmaiplaces.net
Is Bromine Water An Oxidising Agent? Trust The Answer Bromine Water Reducing Agent Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. The reducing agent is the species that is being oxidized (losing electrons). In the process, bromide ions are oxidized to bromine. Bromide reduces sulfuric acid to sulfur dioxide. A reducing agent is typically in one of its lower possible. Bromine Water Reducing Agent.
From www.spaparts.com
Bromine Water Treatment by Natural Chemistry Spa Parts + by Allied Bromine Water Reducing Agent A reducing agent is oxidized,. The brown colour of bromine water turns colourless because bromine molecules are reduced to become bromide ions. The reducing reagent in this reaction is ca. Identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing agents in redox reactions. The reducing agent is the species that is being oxidized (losing. Bromine Water Reducing Agent.
From www.indiamart.com
Bromine Water, Laboratory, 500ml Bottle at Rs 413 in Hyderabad ID Bromine Water Reducing Agent Bromide reduces sulfuric acid to sulfur dioxide. The reducing agent undergoes oxidation with the loss of electrons. The reducing agent is the species that is being oxidized (losing electrons). So, the carbon electrode that is immersed in the solution of the reducing agent becomes the negative cell. A reducing agent is typically in one of its lower possible oxidation states,. Bromine Water Reducing Agent.
From www.slideshare.net
Oxidation & reduction Bromine Water Reducing Agent The reducing reagent in this reaction is ca. Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. The reducing agent undergoes oxidation with the loss of electrons. Identify. Bromine Water Reducing Agent.
From www.youtube.com
Bromine Water Test Explained// HSC Chemistry YouTube Bromine Water Reducing Agent Bromide reduces sulfuric acid to sulfur dioxide. Identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing agents in redox reactions. A reducing agent is oxidized,. The reducing agent undergoes oxidation with the loss of electrons. Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide. Bromine Water Reducing Agent.
From www.indiamart.com
Nice B32229 Bromine Water, 500ml at Rs 319 Motijheel North 24 Bromine Water Reducing Agent So, the carbon electrode that is immersed in the solution of the reducing agent becomes the negative cell. The reducing reagent in this reaction is ca. The brown colour of bromine water turns colourless because bromine molecules are reduced to become bromide ions. A reducing agent is oxidized,. Identify how to view standard reduction potentials from the perspective of viable. Bromine Water Reducing Agent.
From www.slideshare.net
Carbohydrate 1 Bromine Water Reducing Agent The reducing reagent in this reaction is ca. A reducing agent is oxidized,. The reducing agent is the species that is being oxidized (losing electrons). A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. In the process, bromide ions are oxidized to bromine. The reducing agent undergoes oxidation with. Bromine Water Reducing Agent.
From www.numerade.com
SOLVEDBromine is obtained from sea water by the following reaction Bromine Water Reducing Agent The reducing agent is the species that is being oxidized (losing electrons). So, the carbon electrode that is immersed in the solution of the reducing agent becomes the negative cell. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Bromine molecules, which give the bromine water its brown colour,. Bromine Water Reducing Agent.
From www.indiamart.com
ACS Liquid Bromine Water, For Industry, Grade Standard Extra Pure at Bromine Water Reducing Agent Bromide reduces sulfuric acid to sulfur dioxide. The reducing agent undergoes oxidation with the loss of electrons. Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. So, the carbon electrode that is immersed in the solution of the reducing agent becomes the negative cell. In the process, bromide. Bromine Water Reducing Agent.
From vattukhoahoc.com
Bromine water Br2 Dung dịch Brom Bromine Water Reducing Agent A reducing agent is oxidized,. The reducing reagent in this reaction is ca. The reducing agent is the species that is being oxidized (losing electrons). The brown colour of bromine water turns colourless because bromine molecules are reduced to become bromide ions. So, the carbon electrode that is immersed in the solution of the reducing agent becomes the negative cell.. Bromine Water Reducing Agent.
From www.arkanlabs.com
Bromine Water 500 ML Bromine Water Reducing Agent Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. Identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing agents in redox reactions. The reducing agent is the species that is being oxidized (losing electrons). The reducing reagent in this reaction is ca.. Bromine Water Reducing Agent.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation Bromine Water Reducing Agent Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. So, the carbon electrode that is immersed in the solution of the reducing agent becomes the negative cell. The. Bromine Water Reducing Agent.
From slideplayer.com
1.5 b Learning apply knowledge of oxidation and reduction to Bromine Water Reducing Agent So, the carbon electrode that is immersed in the solution of the reducing agent becomes the negative cell. Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. The reducing agent undergoes oxidation with the loss of electrons. Identify how to view standard reduction potentials from the perspective of. Bromine Water Reducing Agent.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation Bromine Water Reducing Agent In the process, bromide ions are oxidized to bromine. The reducing reagent in this reaction is ca. Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. So, the carbon electrode that is immersed in the solution of the reducing agent becomes the negative cell. A reducing agent is. Bromine Water Reducing Agent.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Ammonia and Bromine Water Reducing Agent A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. A reducing agent is oxidized,. The reducing agent is the species that is being oxidized (losing electrons). Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. In the. Bromine Water Reducing Agent.
From www.indiamart.com
Bromine Water at Rs 3250 Bromine Water in Jodhpur ID 21546942012 Bromine Water Reducing Agent The reducing reagent in this reaction is ca. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. In the process, bromide ions are oxidized to bromine. Bromine molecules, which give the bromine water its brown colour, gain the electrons and are reduced to colourless bromide ions, br. The reducing. Bromine Water Reducing Agent.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation Bromine Water Reducing Agent In the process, bromide ions are oxidized to bromine. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. The reducing agent undergoes oxidation with the loss of electrons. Identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing agents in redox reactions. The brown. Bromine Water Reducing Agent.