What Is The Principal Quantum Number N For The 3D Orbital . In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers assigned to each electron in an atom. Orbitals for which n = 2 are larger than those for which n = 1, for example. The principal quantum number (n) describes the size of the orbital. It is the number that governs all the other quantum numbers. The pauli exclusion principle means that no two electrons can share the same quantum numbers. The letter in the orbital name defines. What are the values for the four quantum numbers of an electron in the 3d orbital? Quantum numbers provide important information about the energy and spatial distribution of an electron. N is the principle quantum number. $n$, the principle quantum number defines the. The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. Because they have opposite electrical charges,. The value of the principal quantum number n is the level of the principal electronic shell (principal level). And #m_l# goes from l. All orbitals that have the same n value are in the same principal level.
from www.technocrazed.com
All orbitals that have the same n value are in the same principal level. The letter in the orbital name defines. In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers assigned to each electron in an atom. The principal quantum number (n) describes the size of the orbital. And #m_l# goes from l. Because they have opposite electrical charges,. Orbitals for which n = 2 are larger than those for which n = 1, for example. The value of the principal quantum number n is the level of the principal electronic shell (principal level). Quantum numbers provide important information about the energy and spatial distribution of an electron. The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n.
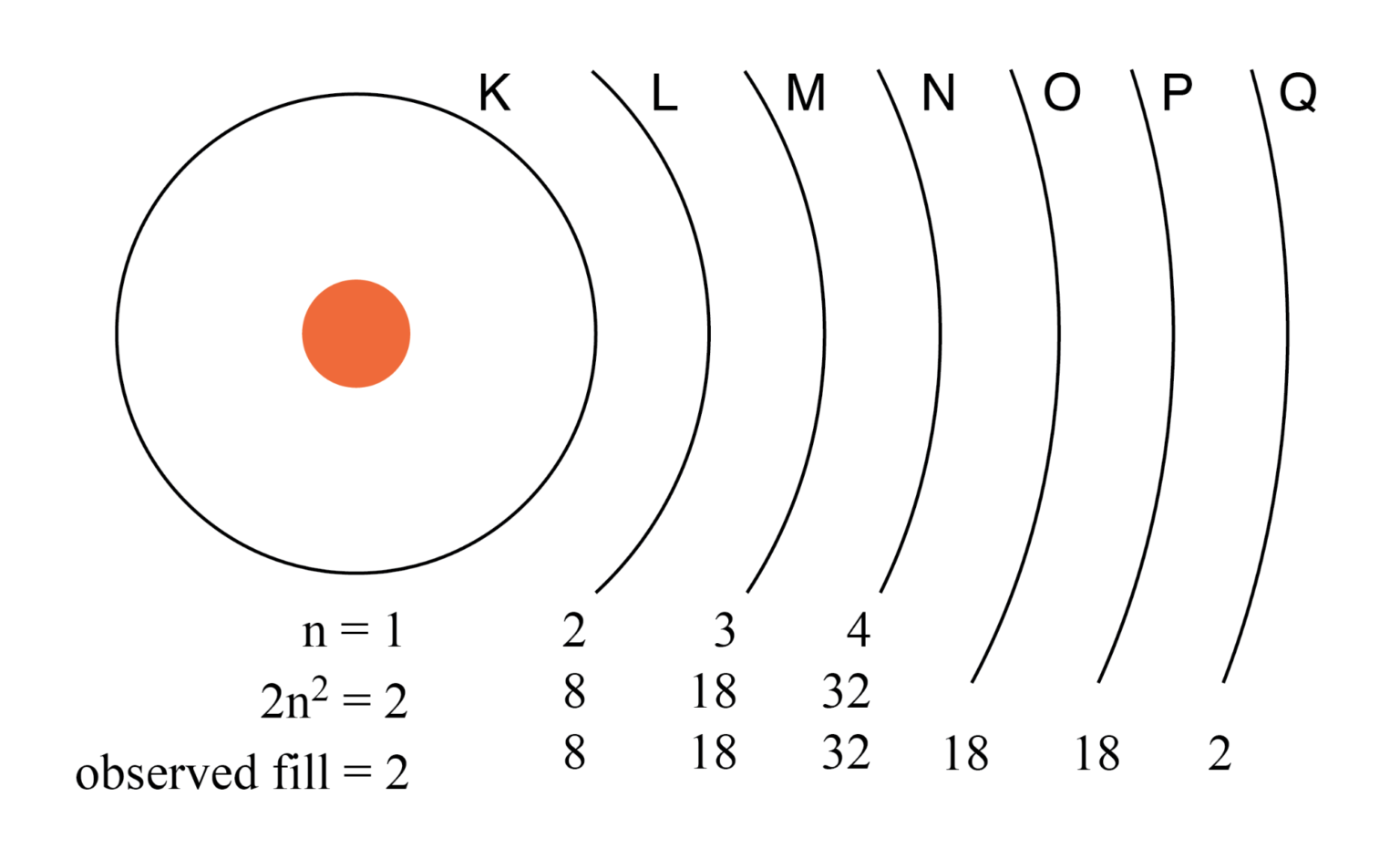
Principal quantum number n and maximum number of electrons per shell
What Is The Principal Quantum Number N For The 3D Orbital N is the principle quantum number. Orbitals for which n = 2 are larger than those for which n = 1, for example. Quantum numbers provide important information about the energy and spatial distribution of an electron. $n$, the principle quantum number defines the. All orbitals that have the same n value are in the same principal level. The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. What are the values for the four quantum numbers of an electron in the 3d orbital? The pauli exclusion principle means that no two electrons can share the same quantum numbers. It is the number that governs all the other quantum numbers. N is the principle quantum number. The letter in the orbital name defines. Because they have opposite electrical charges,. And #m_l# goes from l. The value of the principal quantum number n is the level of the principal electronic shell (principal level). In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers assigned to each electron in an atom. The principal quantum number (n) describes the size of the orbital.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID3181772 What Is The Principal Quantum Number N For The 3D Orbital It is the number that governs all the other quantum numbers. All orbitals that have the same n value are in the same principal level. The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. $n$, the principle quantum number defines the. Orbitals for which n = 2 are larger. What Is The Principal Quantum Number N For The 3D Orbital.
From www.youtube.com
What is the total number of orbitals associated with the principal What Is The Principal Quantum Number N For The 3D Orbital The principal quantum number (n) describes the size of the orbital. It is the number that governs all the other quantum numbers. In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers assigned to each electron in an atom. Orbitals for which n = 2 are larger than those for which n = 1, for. What Is The Principal Quantum Number N For The 3D Orbital.
From www.youtube.com
Principal quantum number (n) & Orbital angular momentum (l) The What Is The Principal Quantum Number N For The 3D Orbital The letter in the orbital name defines. And #m_l# goes from l. It is the number that governs all the other quantum numbers. Because they have opposite electrical charges,. Quantum numbers provide important information about the energy and spatial distribution of an electron. In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers assigned to. What Is The Principal Quantum Number N For The 3D Orbital.
From www.technocrazed.com
Principal quantum number n and maximum number of electrons per shell What Is The Principal Quantum Number N For The 3D Orbital Because they have opposite electrical charges,. Quantum numbers provide important information about the energy and spatial distribution of an electron. In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers assigned to each electron in an atom. The value of the principal quantum number n is the level of the principal electronic shell (principal level).. What Is The Principal Quantum Number N For The 3D Orbital.
From psiberg.com
What Does Principal Quantum Number Determine? PSIBERG What Is The Principal Quantum Number N For The 3D Orbital Because they have opposite electrical charges,. The pauli exclusion principle means that no two electrons can share the same quantum numbers. The letter in the orbital name defines. Orbitals for which n = 2 are larger than those for which n = 1, for example. What are the values for the four quantum numbers of an electron in the 3d. What Is The Principal Quantum Number N For The 3D Orbital.
From www.researchgate.net
Principal quantum number, types and number of orbitals, maximum number What Is The Principal Quantum Number N For The 3D Orbital $n$, the principle quantum number defines the. The letter in the orbital name defines. The principal quantum number (n) describes the size of the orbital. Orbitals for which n = 2 are larger than those for which n = 1, for example. Because they have opposite electrical charges,. In quantum mechanics, the principal quantum number (symbolized n) is one of. What Is The Principal Quantum Number N For The 3D Orbital.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps What Is The Principal Quantum Number N For The 3D Orbital The principal quantum number (n) describes the size of the orbital. The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers assigned to each electron in an atom. The pauli exclusion principle means that no. What Is The Principal Quantum Number N For The 3D Orbital.
From saylordotorg.github.io
Quantum Numbers for Electrons What Is The Principal Quantum Number N For The 3D Orbital The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. The letter in the orbital name defines. It is the number that governs all the other quantum numbers. In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers assigned to each electron in an atom.. What Is The Principal Quantum Number N For The 3D Orbital.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition What Is The Principal Quantum Number N For The 3D Orbital Quantum numbers provide important information about the energy and spatial distribution of an electron. In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers assigned to each electron in an atom. The pauli exclusion principle means that no two electrons can share the same quantum numbers. It is the number that governs all the other. What Is The Principal Quantum Number N For The 3D Orbital.
From www.youtube.com
Structure of Atom Quantum Numbers Principal Quantum Number YouTube What Is The Principal Quantum Number N For The 3D Orbital Orbitals for which n = 2 are larger than those for which n = 1, for example. The letter in the orbital name defines. N is the principle quantum number. It is the number that governs all the other quantum numbers. The pauli exclusion principle means that no two electrons can share the same quantum numbers. The number before the. What Is The Principal Quantum Number N For The 3D Orbital.
From www.youtube.com
Orbitals, Quantum Numbers & Electron Configuration Multiple Choice What Is The Principal Quantum Number N For The 3D Orbital Orbitals for which n = 2 are larger than those for which n = 1, for example. All orbitals that have the same n value are in the same principal level. The value of the principal quantum number n is the level of the principal electronic shell (principal level). $n$, the principle quantum number defines the. N is the principle. What Is The Principal Quantum Number N For The 3D Orbital.
From www.slideserve.com
PPT Quantum Mechanical Model of the Atom PowerPoint Presentation ID What Is The Principal Quantum Number N For The 3D Orbital All orbitals that have the same n value are in the same principal level. And #m_l# goes from l. N is the principle quantum number. The letter in the orbital name defines. Quantum numbers provide important information about the energy and spatial distribution of an electron. Orbitals for which n = 2 are larger than those for which n =. What Is The Principal Quantum Number N For The 3D Orbital.
From www.slideserve.com
PPT Chapter 5 Electrons In Atoms PowerPoint Presentation, free What Is The Principal Quantum Number N For The 3D Orbital The principal quantum number (n) describes the size of the orbital. In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers assigned to each electron in an atom. $n$, the principle quantum number defines the. The pauli exclusion principle means that no two electrons can share the same quantum numbers. All orbitals that have the. What Is The Principal Quantum Number N For The 3D Orbital.
From www.slideserve.com
PPT Chapter 5 Electrons in Atoms PowerPoint Presentation, free What Is The Principal Quantum Number N For The 3D Orbital And #m_l# goes from l. The pauli exclusion principle means that no two electrons can share the same quantum numbers. It is the number that governs all the other quantum numbers. The principal quantum number (n) describes the size of the orbital. N is the principle quantum number. The letter in the orbital name defines. The number before the orbital. What Is The Principal Quantum Number N For The 3D Orbital.
From techal.org
Principal Quantum Number (n) Techal What Is The Principal Quantum Number N For The 3D Orbital Orbitals for which n = 2 are larger than those for which n = 1, for example. Because they have opposite electrical charges,. All orbitals that have the same n value are in the same principal level. And #m_l# goes from l. N is the principle quantum number. The value of the principal quantum number n is the level of. What Is The Principal Quantum Number N For The 3D Orbital.
From www.slideserve.com
PPT Atomic Theory PowerPoint Presentation ID6909895 What Is The Principal Quantum Number N For The 3D Orbital Because they have opposite electrical charges,. It is the number that governs all the other quantum numbers. The letter in the orbital name defines. In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers assigned to each electron in an atom. N is the principle quantum number. All orbitals that have the same n value. What Is The Principal Quantum Number N For The 3D Orbital.
From www.researchgate.net
The principal quantum number n and the sequence of azimuthal quantum What Is The Principal Quantum Number N For The 3D Orbital The pauli exclusion principle means that no two electrons can share the same quantum numbers. Because they have opposite electrical charges,. In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers assigned to each electron in an atom. The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal. What Is The Principal Quantum Number N For The 3D Orbital.
From www.chemistrylearner.com
Principal Quantum Number Definition, Determination, & Value What Is The Principal Quantum Number N For The 3D Orbital Orbitals for which n = 2 are larger than those for which n = 1, for example. Because they have opposite electrical charges,. N is the principle quantum number. $n$, the principle quantum number defines the. What are the values for the four quantum numbers of an electron in the 3d orbital? And #m_l# goes from l. The letter in. What Is The Principal Quantum Number N For The 3D Orbital.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin What Is The Principal Quantum Number N For The 3D Orbital The principal quantum number (n) describes the size of the orbital. It is the number that governs all the other quantum numbers. The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. What are the values for the four quantum numbers of an electron in the 3d orbital? And #m_l#. What Is The Principal Quantum Number N For The 3D Orbital.
From www.researchgate.net
The principal quantum number n and the sequence of azimuthal quantum What Is The Principal Quantum Number N For The 3D Orbital The pauli exclusion principle means that no two electrons can share the same quantum numbers. N is the principle quantum number. The letter in the orbital name defines. Because they have opposite electrical charges,. What are the values for the four quantum numbers of an electron in the 3d orbital? Quantum numbers provide important information about the energy and spatial. What Is The Principal Quantum Number N For The 3D Orbital.
From techal.org
Principal Quantum Number (n) Techal What Is The Principal Quantum Number N For The 3D Orbital The principal quantum number (n) describes the size of the orbital. What are the values for the four quantum numbers of an electron in the 3d orbital? Orbitals for which n = 2 are larger than those for which n = 1, for example. It is the number that governs all the other quantum numbers. The letter in the orbital. What Is The Principal Quantum Number N For The 3D Orbital.
From www.meta-synthesis.com
Quantum Number Periodic Table Chemogenesis What Is The Principal Quantum Number N For The 3D Orbital The pauli exclusion principle means that no two electrons can share the same quantum numbers. The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. The letter in the orbital name defines. The principal quantum number (n) describes the size of the orbital. It is the number that governs all. What Is The Principal Quantum Number N For The 3D Orbital.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation ID2135857 What Is The Principal Quantum Number N For The 3D Orbital $n$, the principle quantum number defines the. What are the values for the four quantum numbers of an electron in the 3d orbital? N is the principle quantum number. All orbitals that have the same n value are in the same principal level. Quantum numbers provide important information about the energy and spatial distribution of an electron. The letter in. What Is The Principal Quantum Number N For The 3D Orbital.
From byjus.com
What is the total number of orbitalsassociated with the principal What Is The Principal Quantum Number N For The 3D Orbital Quantum numbers provide important information about the energy and spatial distribution of an electron. The letter in the orbital name defines. The principal quantum number (n) describes the size of the orbital. The value of the principal quantum number n is the level of the principal electronic shell (principal level). Orbitals for which n = 2 are larger than those. What Is The Principal Quantum Number N For The 3D Orbital.
From www.slideserve.com
PPT Quantum numbers and orbital energies Each atom’s electron has a What Is The Principal Quantum Number N For The 3D Orbital The principal quantum number (n) describes the size of the orbital. And #m_l# goes from l. The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. Orbitals for which n = 2 are larger than those for which n = 1, for example. Because they have opposite electrical charges,. The. What Is The Principal Quantum Number N For The 3D Orbital.
From www.priyamstudycentre.com
Quantum Number Orbitals Diagram, Definition, Chart, Shape What Is The Principal Quantum Number N For The 3D Orbital The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. And #m_l# goes from l. In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers assigned to each electron in an atom. Orbitals for which n = 2 are larger than those for which n. What Is The Principal Quantum Number N For The 3D Orbital.
From www.vrogue.co
Quantum Number Orbital Definition Formula Diagram Sha vrogue.co What Is The Principal Quantum Number N For The 3D Orbital It is the number that governs all the other quantum numbers. $n$, the principle quantum number defines the. The pauli exclusion principle means that no two electrons can share the same quantum numbers. All orbitals that have the same n value are in the same principal level. The letter in the orbital name defines. Quantum numbers provide important information about. What Is The Principal Quantum Number N For The 3D Orbital.
From techal.org
Principal Quantum Number (n) Techal What Is The Principal Quantum Number N For The 3D Orbital N is the principle quantum number. Quantum numbers provide important information about the energy and spatial distribution of an electron. Because they have opposite electrical charges,. The letter in the orbital name defines. And #m_l# goes from l. The pauli exclusion principle means that no two electrons can share the same quantum numbers. The principal quantum number (n) describes the. What Is The Principal Quantum Number N For The 3D Orbital.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID6766216 What Is The Principal Quantum Number N For The 3D Orbital The value of the principal quantum number n is the level of the principal electronic shell (principal level). And #m_l# goes from l. All orbitals that have the same n value are in the same principal level. The principal quantum number (n) describes the size of the orbital. The letter in the orbital name defines. What are the values for. What Is The Principal Quantum Number N For The 3D Orbital.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition What Is The Principal Quantum Number N For The 3D Orbital All orbitals that have the same n value are in the same principal level. N is the principle quantum number. The letter in the orbital name defines. $n$, the principle quantum number defines the. The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. Because they have opposite electrical charges,.. What Is The Principal Quantum Number N For The 3D Orbital.
From www.youtube.com
The Principal Quantum Number (n) YouTube What Is The Principal Quantum Number N For The 3D Orbital It is the number that governs all the other quantum numbers. Orbitals for which n = 2 are larger than those for which n = 1, for example. Quantum numbers provide important information about the energy and spatial distribution of an electron. The pauli exclusion principle means that no two electrons can share the same quantum numbers. And #m_l# goes. What Is The Principal Quantum Number N For The 3D Orbital.
From www.slideserve.com
PPT Quantum Numbers and Electronic Configuration PowerPoint What Is The Principal Quantum Number N For The 3D Orbital It is the number that governs all the other quantum numbers. The pauli exclusion principle means that no two electrons can share the same quantum numbers. The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. And #m_l# goes from l. $n$, the principle quantum number defines the. Quantum numbers. What Is The Principal Quantum Number N For The 3D Orbital.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition What Is The Principal Quantum Number N For The 3D Orbital In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers assigned to each electron in an atom. The principal quantum number (n) describes the size of the orbital. Orbitals for which n = 2 are larger than those for which n = 1, for example. All orbitals that have the same n value are in. What Is The Principal Quantum Number N For The 3D Orbital.
From www.pearson.com
Identify each of the following orbitals, and give n and l quantum What Is The Principal Quantum Number N For The 3D Orbital The value of the principal quantum number n is the level of the principal electronic shell (principal level). The letter in the orbital name defines. $n$, the principle quantum number defines the. Orbitals for which n = 2 are larger than those for which n = 1, for example. Because they have opposite electrical charges,. In quantum mechanics, the principal. What Is The Principal Quantum Number N For The 3D Orbital.
From mavink.com
Quantum Numbers And Orbital Shapes What Is The Principal Quantum Number N For The 3D Orbital The principal quantum number (n) describes the size of the orbital. The value of the principal quantum number n is the level of the principal electronic shell (principal level). All orbitals that have the same n value are in the same principal level. And #m_l# goes from l. Because they have opposite electrical charges,. $n$, the principle quantum number defines. What Is The Principal Quantum Number N For The 3D Orbital.