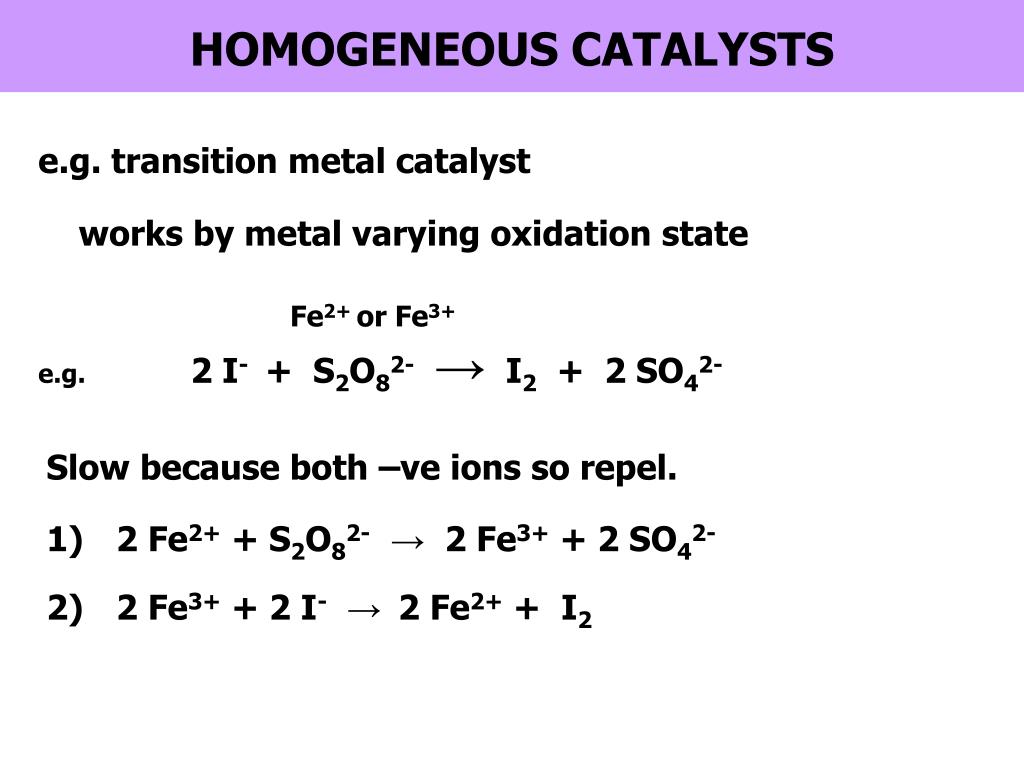
Catalyst Example Equation . catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Adding a drop of blood to a solution of 30% hydrogen. Another example is the use of solid iron, fe, in the haber process for making ammonia. After the reaction occurs, a catalyst returns to its original state; A catalyst is a substance that is used to speed up a chemical reaction but it is not. for example, vanadium (v) oxide, v 2 o 5, is a solid catalyst used in the contact process for making sulfuric acid. Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to proceed via a. In this type of catalysis. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids;
from www.slideserve.com
Catalysts participate in a chemical reaction and increase its rate. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Another example is the use of solid iron, fe, in the haber process for making ammonia. After the reaction occurs, a catalyst returns to its original state; for example, vanadium (v) oxide, v 2 o 5, is a solid catalyst used in the contact process for making sulfuric acid. Catalysts allow a reaction to proceed via a. A catalyst is a substance that is used to speed up a chemical reaction but it is not. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In this type of catalysis.
PPT Starter 1)Definition of catalysts 2) Difference between
Catalyst Example Equation Catalysts participate in a chemical reaction and increase its rate. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; After the reaction occurs, a catalyst returns to its original state; Catalysts participate in a chemical reaction and increase its rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that is used to speed up a chemical reaction but it is not. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. for example, vanadium (v) oxide, v 2 o 5, is a solid catalyst used in the contact process for making sulfuric acid. In this type of catalysis. They do not appear in the reaction’s net equation and are not consumed during the reaction. Adding a drop of blood to a solution of 30% hydrogen. Another example is the use of solid iron, fe, in the haber process for making ammonia. Catalysts allow a reaction to proceed via a.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Example Equation for example, vanadium (v) oxide, v 2 o 5, is a solid catalyst used in the contact process for making sulfuric acid. Another example is the use of solid iron, fe, in the haber process for making ammonia. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being. Catalyst Example Equation.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalyst Example Equation A catalyst is a substance that is used to speed up a chemical reaction but it is not. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; They do not appear in the reaction’s net equation and are not consumed during the reaction. for example, vanadium (v) oxide, v 2 o. Catalyst Example Equation.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Example Equation Another example is the use of solid iron, fe, in the haber process for making ammonia. Adding a drop of blood to a solution of 30% hydrogen. Catalysts participate in a chemical reaction and increase its rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In this type of catalysis. After. Catalyst Example Equation.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalyst Example Equation Catalysts allow a reaction to proceed via a. They do not appear in the reaction’s net equation and are not consumed during the reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that is used to speed up a chemical reaction but it is not. . Catalyst Example Equation.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Example Equation the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Another example is the use of solid iron, fe, in the haber process for making ammonia. In this type of catalysis. Adding a drop of blood to a solution of 30% hydrogen. After the reaction occurs, a catalyst returns to its original state;. Catalyst Example Equation.
From 45.153.231.124
Chemical Catalyst Definition Reaction Types And Examples Gambaran Catalyst Example Equation catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. After the reaction occurs, a catalyst returns to its original state; Another example is the use of. Catalyst Example Equation.
From shapeguidance1.gitlab.io
Outrageous Does A Catalyst Increase The Rate Of Reaction Year 12 Catalyst Example Equation catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Adding a drop of blood to a solution of 30% hydrogen. Catalysts participate in a chemical reaction and increase its rate. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used. Catalyst Example Equation.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Example Equation A catalyst is a substance that is used to speed up a chemical reaction but it is not. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. for example, vanadium (v) oxide, v 2 o 5, is a solid catalyst used in the contact process for making sulfuric acid. They do. Catalyst Example Equation.
From www.pinterest.com
Heterogeneous Catalyst Easy Science Easy science, Chemical equation Catalyst Example Equation Another example is the use of solid iron, fe, in the haber process for making ammonia. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Catalysts participate in a chemical reaction and increase its rate. After the reaction occurs, a catalyst returns to its original state; A catalyst is a substance that. Catalyst Example Equation.
From www.hotzxgirl.com
Enzyme Catalysis Study Material For Iit Jee Askiitians Hot Sex Picture Catalyst Example Equation After the reaction occurs, a catalyst returns to its original state; Catalysts participate in a chemical reaction and increase its rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Another example is the use of solid iron, fe, in the haber process for making ammonia. Catalysts allow a reaction to proceed. Catalyst Example Equation.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent Catalyst Example Equation After the reaction occurs, a catalyst returns to its original state; In this type of catalysis. Another example is the use of solid iron, fe, in the haber process for making ammonia. for example, vanadium (v) oxide, v 2 o 5, is a solid catalyst used in the contact process for making sulfuric acid. catalyst, in chemistry, any. Catalyst Example Equation.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation ID591293 Catalyst Example Equation catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that is used to speed up a chemical reaction but it is not. for example, vanadium (v) oxide, v 2 o 5, is a solid catalyst used in the contact process for making sulfuric acid. a. Catalyst Example Equation.
From wzhessaykuo.web.fc2.com
How to write catalyst in chemical equation Catalyst Example Equation Adding a drop of blood to a solution of 30% hydrogen. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. After the reaction occurs, a catalyst returns to its original state; a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being. Catalyst Example Equation.
From www.slideserve.com
PPT Heterogeneous Catalysis & Solid State Physics PowerPoint Catalyst Example Equation Catalysts allow a reaction to proceed via a. They do not appear in the reaction’s net equation and are not consumed during the reaction. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. catalyst, in chemistry, any substance that increases the rate. Catalyst Example Equation.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalyst Example Equation In this type of catalysis. for example, vanadium (v) oxide, v 2 o 5, is a solid catalyst used in the contact process for making sulfuric acid. Another example is the use of solid iron, fe, in the haber process for making ammonia. Catalysts participate in a chemical reaction and increase its rate. a catalyst is a substance. Catalyst Example Equation.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Catalyst Example Equation Another example is the use of solid iron, fe, in the haber process for making ammonia. They do not appear in the reaction’s net equation and are not consumed during the reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the most effective catalyst of all is the enzyme catalase,. Catalyst Example Equation.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Example Equation Catalysts allow a reaction to proceed via a. After the reaction occurs, a catalyst returns to its original state; Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself. Catalyst Example Equation.
From www.youtube.com
Phase Transfer Catalyst Quaternary Ammonium Salt Organic Chemistry Catalyst Example Equation Catalysts participate in a chemical reaction and increase its rate. Adding a drop of blood to a solution of 30% hydrogen. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Catalysts allow a reaction to proceed via a. In this type of catalysis. Another example is the use of solid iron, fe,. Catalyst Example Equation.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Example Equation Catalysts allow a reaction to proceed via a. Another example is the use of solid iron, fe, in the haber process for making ammonia. In this type of catalysis. After the reaction occurs, a catalyst returns to its original state; a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without. Catalyst Example Equation.
From www.youtube.com
Identifying Catalyst and Intermediates in Elementary Steps of Catalyst Example Equation for example, vanadium (v) oxide, v 2 o 5, is a solid catalyst used in the contact process for making sulfuric acid. In this type of catalysis. Catalysts participate in a chemical reaction and increase its rate. After the reaction occurs, a catalyst returns to its original state; Another example is the use of solid iron, fe, in the. Catalyst Example Equation.
From www.pinterest.com
Catalyst Easy Science in 2022 Energy activities, Chemical reactions Catalyst Example Equation They do not appear in the reaction’s net equation and are not consumed during the reaction. Adding a drop of blood to a solution of 30% hydrogen. for example, vanadium (v) oxide, v 2 o 5, is a solid catalyst used in the contact process for making sulfuric acid. Catalysts allow a reaction to proceed via a. catalyst,. Catalyst Example Equation.
From www.slideshare.net
Catalysis Catalyst Example Equation Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. After the reaction occurs, a catalyst returns to its original state; A catalyst is a substance that is used to speed up a chemical reaction but it is not. catalyst, in chemistry, any. Catalyst Example Equation.
From www.youtube.com
01.10 Base Catalysis and Summary YouTube Catalyst Example Equation After the reaction occurs, a catalyst returns to its original state; catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Another example is the use of solid iron, fe, in the haber process for making ammonia. a catalyst is a substance that increases the rate of a chemical reaction by lowering. Catalyst Example Equation.
From www.youtube.com
Practice Identifying Catalysts and Intermediates YouTube Catalyst Example Equation They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a chemical reaction and increase its rate. After the reaction occurs, a catalyst returns to its original state; Adding a drop of blood to a solution of 30% hydrogen. In this type of catalysis. for example, vanadium (v) oxide, v. Catalyst Example Equation.
From www.nagwa.com
Question Video Identifying the Name of a Catalyst That Slows Down a Catalyst Example Equation Adding a drop of blood to a solution of 30% hydrogen. In this type of catalysis. for example, vanadium (v) oxide, v 2 o 5, is a solid catalyst used in the contact process for making sulfuric acid. After the reaction occurs, a catalyst returns to its original state; the most effective catalyst of all is the enzyme. Catalyst Example Equation.
From brainly.in
define catalyst with example ?? Brainly.in Catalyst Example Equation Catalysts allow a reaction to proceed via a. In this type of catalysis. Another example is the use of solid iron, fe, in the haber process for making ammonia. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that is used to speed up a chemical reaction. Catalyst Example Equation.
From www.sciencelearn.org.nz
Catalytic converter catalyst — Science Learning Hub Catalyst Example Equation Catalysts participate in a chemical reaction and increase its rate. Catalysts allow a reaction to proceed via a. A catalyst is a substance that is used to speed up a chemical reaction but it is not. In this type of catalysis. for example, vanadium (v) oxide, v 2 o 5, is a solid catalyst used in the contact process. Catalyst Example Equation.
From www.researchgate.net
Heterogeneous Catalysis and Electrocatalysis Tests of the Catalysts (A Catalyst Example Equation a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Adding a drop of blood to a solution of 30% hydrogen. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a chemical reaction and. Catalyst Example Equation.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2683737 Catalyst Example Equation Another example is the use of solid iron, fe, in the haber process for making ammonia. A catalyst is a substance that is used to speed up a chemical reaction but it is not. In this type of catalysis. Adding a drop of blood to a solution of 30% hydrogen. They do not appear in the reaction’s net equation and. Catalyst Example Equation.
From www.youtube.com
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube Catalyst Example Equation the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Adding a drop of blood to a solution of 30% hydrogen. for example, vanadium (v) oxide, v 2 o 5, is a solid catalyst used in the contact process for making sulfuric acid. In this type of catalysis. They do not appear. Catalyst Example Equation.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Example Equation In this type of catalysis. A catalyst is a substance that is used to speed up a chemical reaction but it is not. Catalysts participate in a chemical reaction and increase its rate. After the reaction occurs, a catalyst returns to its original state; a catalyst is a substance that increases the rate of a chemical reaction by lowering. Catalyst Example Equation.
From www.slideserve.com
PPT Homogeneous catalysis PowerPoint Presentation, free download ID Catalyst Example Equation A catalyst is a substance that is used to speed up a chemical reaction but it is not. In this type of catalysis. for example, vanadium (v) oxide, v 2 o 5, is a solid catalyst used in the contact process for making sulfuric acid. After the reaction occurs, a catalyst returns to its original state; Catalysts participate in. Catalyst Example Equation.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst Example Equation Catalysts participate in a chemical reaction and increase its rate. Another example is the use of solid iron, fe, in the haber process for making ammonia. for example, vanadium (v) oxide, v 2 o 5, is a solid catalyst used in the contact process for making sulfuric acid. a catalyst is a substance that increases the rate of. Catalyst Example Equation.
From www.youtube.com
16.1 Catalysts (HL) YouTube Catalyst Example Equation Catalysts allow a reaction to proceed via a. They do not appear in the reaction’s net equation and are not consumed during the reaction. Another example is the use of solid iron, fe, in the haber process for making ammonia. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without. Catalyst Example Equation.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Catalyst Example Equation In this type of catalysis. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. A catalyst is a substance that is used to speed up a chemical reaction but it is not. They do not appear in the reaction’s net equation and are. Catalyst Example Equation.