Potentiometric Titration Of Hcl And Naoh . They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. there are two basic types of acid base titrations, indicator and potentiometric. standardization of the 0.1 m naoh titrant. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions.
from studylib.net
In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. standardization of the 0.1 m naoh titrant. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. there are two basic types of acid base titrations, indicator and potentiometric. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then.
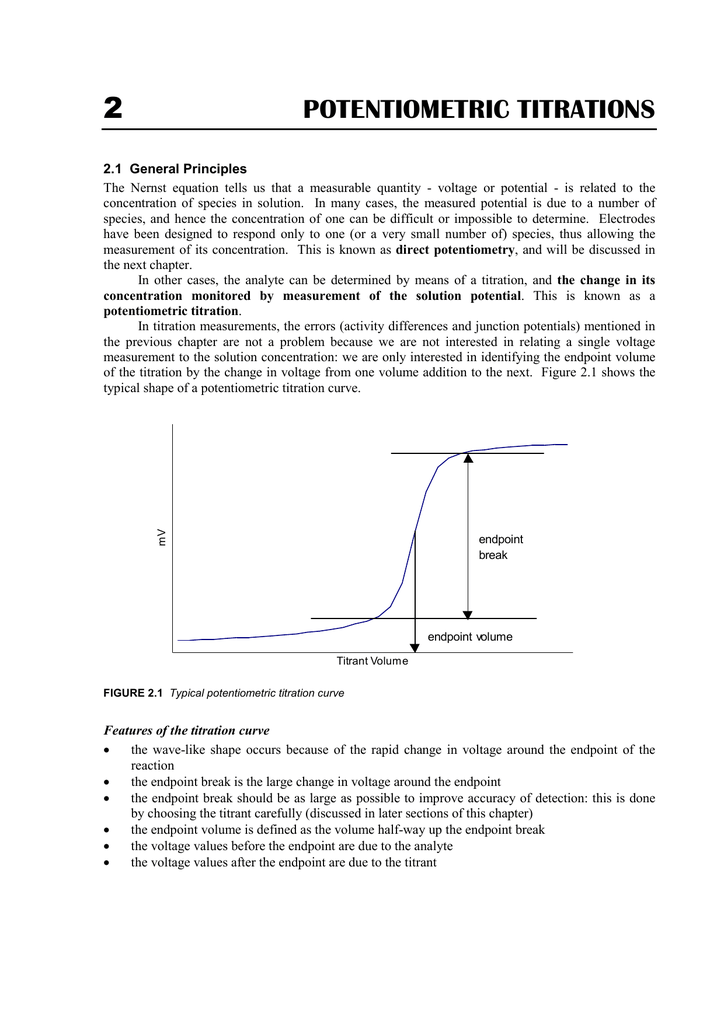
2 POTENTIOMETRIC TITRATIONS
Potentiometric Titration Of Hcl And Naoh in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. there are two basic types of acid base titrations, indicator and potentiometric. standardization of the 0.1 m naoh titrant. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp.
From exotnsiwz.blob.core.windows.net
Titration Of Naoh With Hcl at Katherine Grassi blog Potentiometric Titration Of Hcl And Naoh Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. there are two basic types of acid base titrations, indicator and potentiometric. standardization of the 0.1 m naoh titrant. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce. Potentiometric Titration Of Hcl And Naoh.
From www.studocu.com
Lab 5 Potentiometric Titration Potentiometry Titration of a Halide Potentiometric Titration Of Hcl And Naoh In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. in this experiment, we will prepare sodium hydroxide standard solution that. Potentiometric Titration Of Hcl And Naoh.
From monomole.com
Titration overview Mono Mole Potentiometric Titration Of Hcl And Naoh there are two basic types of acid base titrations, indicator and potentiometric. in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. In this experiment students. Potentiometric Titration Of Hcl And Naoh.
From exomuvqjn.blob.core.windows.net
What Happens When You Mix Hcl And Naoh at Christopher Schiller blog Potentiometric Titration Of Hcl And Naoh there are two basic types of acid base titrations, indicator and potentiometric. standardization of the 0.1 m naoh titrant. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. In this experiment students neutralise sodium hydroxide with hydrochloric. Potentiometric Titration Of Hcl And Naoh.
From www.tutormyself.com
233 Archives TutorMyself Chemistry Potentiometric Titration Of Hcl And Naoh They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. standardization of the 0.1 m naoh titrant. In an indicator based titration you add another chemical that changes color at the ph equal to the. Potentiometric Titration Of Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Potentiometric Titration Of Hcl And Naoh They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. standardization of the 0.1 m naoh titrant. there are two basic types of acid base titrations, indicator and potentiometric. in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. In an indicator based titration you add another. Potentiometric Titration Of Hcl And Naoh.
From dxolxlskr.blob.core.windows.net
Why Use Titration To Find Concentration at Rodney Ruggerio blog Potentiometric Titration Of Hcl And Naoh in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. there are two basic types of acid base titrations, indicator and potentiometric. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. In an indicator based titration you add another chemical that changes color at the ph equal to. Potentiometric Titration Of Hcl And Naoh.
From www.youtube.com
Potentiometric titration between HCl & NaoH YouTube Potentiometric Titration Of Hcl And Naoh in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. standardization. Potentiometric Titration Of Hcl And Naoh.
From www.researchgate.net
3 Potentiometric titration of a solution containing a strong acid (HCl Potentiometric Titration Of Hcl And Naoh In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. there are two basic types of acid base titrations, indicator and potentiometric. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. In this experiment students neutralise. Potentiometric Titration Of Hcl And Naoh.
From dxonsvczs.blob.core.windows.net
Titration Base Concentration at James Cummings blog Potentiometric Titration Of Hcl And Naoh In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. In this experiment. Potentiometric Titration Of Hcl And Naoh.
From www.chemistryscl.com
NaOH and HCl Titration Curves Selecting Indicators Potentiometric Titration Of Hcl And Naoh standardization of the 0.1 m naoh titrant. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. there are two basic types of acid base titrations,. Potentiometric Titration Of Hcl And Naoh.
From www.elementalchemistry.in
ELEMENTAL CHEMISTRY Potentiometric Titrations Potentiometric Titration Of Hcl And Naoh this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. In an indicator based titration you add another chemical that changes color at the ph equal to the. Potentiometric Titration Of Hcl And Naoh.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID737892 Potentiometric Titration Of Hcl And Naoh In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. in this experiment, we will prepare sodium hydroxide standard solution that. Potentiometric Titration Of Hcl And Naoh.
From mungfali.com
Titration Curve HCl And NaOH Potentiometric Titration Of Hcl And Naoh Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. In an indicator based titration you add another chemical that changes color at the ph equal to the. Potentiometric Titration Of Hcl And Naoh.
From www.youtube.com
Conductometric titration (Precipitation titration of KCl vs AgNO3 and Potentiometric Titration Of Hcl And Naoh in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt. Potentiometric Titration Of Hcl And Naoh.
From www.youtube.com
Potentiometric acid base titrations YouTube Potentiometric Titration Of Hcl And Naoh standardization of the 0.1 m naoh titrant. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. there are. Potentiometric Titration Of Hcl And Naoh.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Potentiometric Titration Of Hcl And Naoh In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. this experiment will introduce you to quantitative volumetric analysis and potentiometric. Potentiometric Titration Of Hcl And Naoh.
From pubs.sciepub.com
Figure 6. First derivative plot of the titration of strong acid (HCl= 0 Potentiometric Titration Of Hcl And Naoh Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. there are two basic types of acid base titrations, indicator and potentiometric. In this experiment students. Potentiometric Titration Of Hcl And Naoh.
From pubs.sciepub.com
Figure 5B. Plot of the titration of strong acid (HCl= 0.1M) with strong Potentiometric Titration Of Hcl And Naoh this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. there are. Potentiometric Titration Of Hcl And Naoh.
From www.researchgate.net
Potentiometric titrations of 1.0 mmol·dm −3 [Pt(SMC)(H 2 O) 2 ] + with Potentiometric Titration Of Hcl And Naoh in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. there are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. They then. Potentiometric Titration Of Hcl And Naoh.
From www.youtube.com
conductometric titration strong acid vs. strong base YouTube Potentiometric Titration Of Hcl And Naoh in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. standardization of the 0.1 m naoh titrant. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. In an indicator based titration you add another chemical. Potentiometric Titration Of Hcl And Naoh.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID5410570 Potentiometric Titration Of Hcl And Naoh They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. there are two basic types of acid base titrations, indicator and potentiometric. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. standardization of the 0.1. Potentiometric Titration Of Hcl And Naoh.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Potentiometric Titration Of Hcl And Naoh Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. They. Potentiometric Titration Of Hcl And Naoh.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Potentiometric Titration Of Hcl And Naoh standardization of the 0.1 m naoh titrant. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. there are two basic types of acid base titrations, indicator and potentiometric. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In an indicator based titration you. Potentiometric Titration Of Hcl And Naoh.
From www.youtube.com
Conductometric Titration & Titration Curves // HSC Chemistry YouTube Potentiometric Titration Of Hcl And Naoh In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. standardization of the 0.1 m naoh titrant. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. there are two basic types of acid base titrations, indicator and potentiometric. in this experiment, we. Potentiometric Titration Of Hcl And Naoh.
From studylib.net
2 POTENTIOMETRIC TITRATIONS Potentiometric Titration Of Hcl And Naoh in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. standardization of the 0.1 m naoh titrant. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. Potentiometric Titration Of Hcl And Naoh.
From dokumen.tips
(PDF) Potentiometric Titration of HCl and Na2COgenchem99/doc Potentiometric Titration Of Hcl And Naoh Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. there are two basic types of acid base titrations, indicator and potentiometric. in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. standardization of the 0.1 m naoh titrant. They then concentrate the solution and allow it to. Potentiometric Titration Of Hcl And Naoh.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Potentiometric Titration Of Hcl And Naoh In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. there are two basic types of acid base titrations, indicator and potentiometric. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. In an indicator based titration you add another chemical that changes color at the. Potentiometric Titration Of Hcl And Naoh.
From chemwiki.ucdavis.edu
9.1 Overview of Titrimetry Chemwiki Potentiometric Titration Of Hcl And Naoh there are two basic types of acid base titrations, indicator and potentiometric. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Potentiometric Titration Of Hcl And Naoh.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Potentiometric Titration Of Hcl And Naoh Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. In an indicator based titration you add another chemical that changes color at the. Potentiometric Titration Of Hcl And Naoh.
From www.youtube.com
Titration of HCl with NaOH YouTube Potentiometric Titration Of Hcl And Naoh in this experiment, we will prepare sodium hydroxide standard solution that is standardized with khp. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. standardization of the 0.1 m naoh titrant. Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. In an indicator based titration you add another chemical. Potentiometric Titration Of Hcl And Naoh.
From dxoqraial.blob.core.windows.net
Titration Reaction Of Hcl And Naoh at Lucia Chamberlain blog Potentiometric Titration Of Hcl And Naoh Weigh to the nearest 0.1 mg about 2.5 g of sulfamic acid and then. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. In an indicator based titration you add another chemical that changes color at the ph equal to the. Potentiometric Titration Of Hcl And Naoh.
From www.vrogue.co
Potentiometric Titration Principle Methods And Types vrogue.co Potentiometric Titration Of Hcl And Naoh there are two basic types of acid base titrations, indicator and potentiometric. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. They then concentrate the solution. Potentiometric Titration Of Hcl And Naoh.
From exoyzonai.blob.core.windows.net
Titration Curve Labeled Buffer Region at Craig Johnson blog Potentiometric Titration Of Hcl And Naoh In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. this experiment will introduce you to quantitative volumetric analysis and potentiometric titrations. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric. Potentiometric Titration Of Hcl And Naoh.