Tin Number Of Shell Electrons . Including scores of properties, element names in many languages,. 3rd shell can hold 18 electrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. First, find electrons of tin atom. Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. Electron configuration of tin is [kr] 4d10 5s2 5p2. 2nd shell can hold 8 electrons. In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n². The atomic number of tin represents the total number of electrons of tin. Comprehensive data on the chemical element tin is provided on this page; The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Thus, 1st shell can hold 2 electrons. 4th shell can hold 32 electrons, and so on. This would mean 2 electrons could fit in the first shell, 8 could fit in. In the periodic table, the elements are listed in order of increasing atomic number z.
from mungfali.com
2nd shell can hold 8 electrons. The atomic number of tin represents the total number of electrons of tin. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Number of electrons in shells. First, find electrons of tin atom. Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. Comprehensive data on the chemical element tin is provided on this page; Including scores of properties, element names in many languages,. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. 3rd shell can hold 18 electrons.
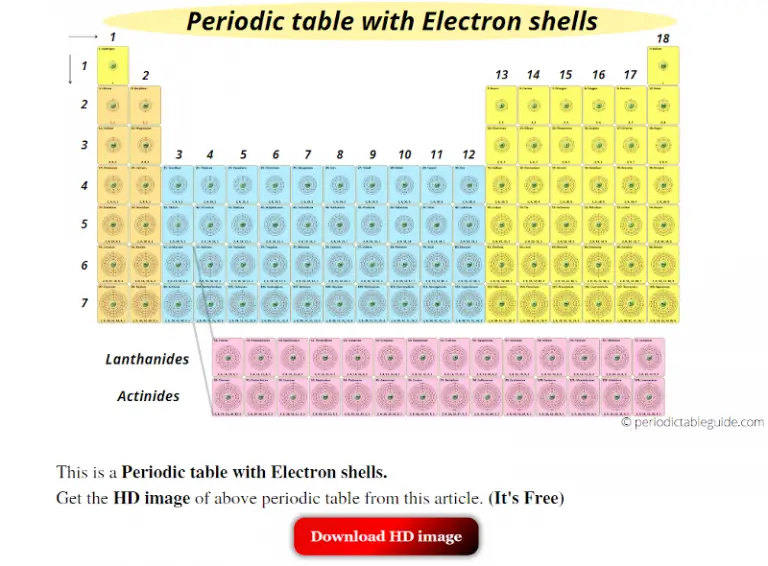
Periodic Table Of Elements Showing Electron Shells
Tin Number Of Shell Electrons Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. 2nd shell can hold 8 electrons. In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n². Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Thus, 1st shell can hold 2 electrons. Electron configuration of tin is [kr] 4d10 5s2 5p2. The atomic number of tin represents the total number of electrons of tin. In the periodic table, the elements are listed in order of increasing atomic number z. First, find electrons of tin atom. 3rd shell can hold 18 electrons. 4th shell can hold 32 electrons, and so on. Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. Number of electrons in shells. Comprehensive data on the chemical element tin is provided on this page; Including scores of properties, element names in many languages,. This would mean 2 electrons could fit in the first shell, 8 could fit in.
From www.alamy.com
Sn Tin, Periodic Table of the Elements, Shell Structure of Tin Tin Number Of Shell Electrons 3rd shell can hold 18 electrons. Electron configuration of tin is [kr] 4d10 5s2 5p2. Including scores of properties, element names in many languages,. In the periodic table, the elements are listed in order of increasing atomic number z. This would mean 2 electrons could fit in the first shell, 8 could fit in. Sources, facts, uses, scarcity (sri), podcasts,. Tin Number Of Shell Electrons.
From utedzz.blogspot.com
Fully Labeled Periodic Table With Names Periodic Table Timeline Tin Number Of Shell Electrons 4th shell can hold 32 electrons, and so on. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. 3rd shell can hold 18 electrons. Including scores of properties, element names in many languages,. Number of electrons in shells. The atomic number of tin represents the. Tin Number Of Shell Electrons.
From www.sciencephoto.com
Tin, atomic structure Stock Image C018/3731 Science Photo Library Tin Number Of Shell Electrons The atomic number of tin represents the total number of electrons of tin. Number of electrons in shells. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Thus, 1st shell can hold 2 electrons. In my textbook, it says that the maximum number of electrons. Tin Number Of Shell Electrons.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Number Of Shell Electrons In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n². Thus, 1st shell can hold 2 electrons. Comprehensive data on the chemical element tin is provided on this page; Number of electrons in shells. In the periodic table, the elements are listed in order of increasing atomic number. Tin Number Of Shell Electrons.
From ar.inspiredpencil.com
Tin Atomic Structure Tin Number Of Shell Electrons Including scores of properties, element names in many languages,. Comprehensive data on the chemical element tin is provided on this page; First, find electrons of tin atom. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 3rd shell can hold 18 electrons. The atomic number of tin represents the total number of electrons of tin. Number of electrons in shells. This. Tin Number Of Shell Electrons.
From www.schoolmykids.com
Tin (Sn) Element Information, Facts, Properties, Uses Periodic Tin Number Of Shell Electrons First, find electrons of tin atom. In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n². 2nd shell can hold 8 electrons. The atomic number of tin represents the total number of electrons of tin. The number of electrons in each element’s electron shells, particularly the outermost valence. Tin Number Of Shell Electrons.
From www.nuclear-power.com
What is Tin Properties of Tin Element Symbol Sn Tin Number Of Shell Electrons 2nd shell can hold 8 electrons. Comprehensive data on the chemical element tin is provided on this page; Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. Including scores of properties, element names in many languages,. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor. Tin Number Of Shell Electrons.
From courses.lumenlearning.com
Organization of Electrons in Atoms Introductory Chemistry Tin Number Of Shell Electrons 4th shell can hold 32 electrons, and so on. 3rd shell can hold 18 electrons. Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. Comprehensive data on the chemical element tin is provided on this page; The atomic number of tin represents the total number of electrons of tin. 2nd shell can hold 8. Tin Number Of Shell Electrons.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell Tin Number Of Shell Electrons Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. 2nd shell can hold 8 electrons. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Number of electrons in shells. Comprehensive data on the chemical element tin is provided on. Tin Number Of Shell Electrons.
From www.youtube.com
How to Find the Valence Electrons for Tin (Sn) YouTube Tin Number Of Shell Electrons 3rd shell can hold 18 electrons. In the periodic table, the elements are listed in order of increasing atomic number z. 2nd shell can hold 8 electrons. This would mean 2 electrons could fit in the first shell, 8 could fit in. In my textbook, it says that the maximum number of electrons that can fit in any given shell. Tin Number Of Shell Electrons.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Tin Number Of Shell Electrons Electron configuration of tin is [kr] 4d10 5s2 5p2. Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. Comprehensive data on the chemical element tin is provided on this page; 3rd shell can hold 18 electrons. First, find electrons of tin atom. This would mean 2 electrons could fit in the first shell, 8. Tin Number Of Shell Electrons.
From mungfali.com
Periodic Table Of Elements Showing Electron Shells Tin Number Of Shell Electrons Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. Electron configuration of tin is [kr] 4d10 5s2 5p2. In the periodic table, the elements are listed in order of increasing atomic number z. 4th shell can hold 32 electrons, and so on. Including scores of properties, element names in many languages,. The atomic number. Tin Number Of Shell Electrons.
From www.britannica.com
Tin Definition, Properties, Uses, & Facts Britannica Tin Number Of Shell Electrons The atomic number of tin represents the total number of electrons of tin. Electron configuration of tin is [kr] 4d10 5s2 5p2. 4th shell can hold 32 electrons, and so on. Number of electrons in shells. In the periodic table, the elements are listed in order of increasing atomic number z. First, find electrons of tin atom. Including scores of. Tin Number Of Shell Electrons.
From en.m.wikipedia.org
FilePeriodic table of elements showing electron shells.png Wikipedia Tin Number Of Shell Electrons In the periodic table, the elements are listed in order of increasing atomic number z. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Number of electrons in shells. 2nd shell can hold 8 electrons. The atomic. Tin Number Of Shell Electrons.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Number Of Shell Electrons Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. Thus, 1st shell can hold 2 electrons. 4th shell can hold 32 electrons, and so on. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Electron configuration of tin is [kr] 4d10 5s2 5p2. In my textbook, it says that the maximum number of electrons that. Tin Number Of Shell Electrons.
From ar.inspiredpencil.com
Periodic Table Electron Shells Tin Number Of Shell Electrons Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. Electron configuration of tin is [kr] 4d10 5s2 5p2. The atomic number of tin represents the total number of electrons of tin. 2nd shell can hold 8 electrons. 4th shell can hold 32 electrons, and so on. This would mean 2 electrons could fit in. Tin Number Of Shell Electrons.
From mungfali.com
Periodic Table Of Elements Showing Electron Shells Tin Number Of Shell Electrons The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. Thus, 1st shell can hold 2 electrons. Comprehensive data on the chemical element tin is provided on this page; The atomic. Tin Number Of Shell Electrons.
From www.britannica.com
Electron shell Definition & Facts Britannica Tin Number Of Shell Electrons Including scores of properties, element names in many languages,. Thus, 1st shell can hold 2 electrons. In the periodic table, the elements are listed in order of increasing atomic number z. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. This would mean 2 electrons. Tin Number Of Shell Electrons.
From www.vectorstock.com
Symbol and electron diagram for tin Royalty Free Vector Tin Number Of Shell Electrons 2nd shell can hold 8 electrons. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. The atomic number of tin represents the total number of electrons of tin. In my textbook, it says that the maximum number of electrons that can fit in any given. Tin Number Of Shell Electrons.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Number Of Shell Electrons Comprehensive data on the chemical element tin is provided on this page; This would mean 2 electrons could fit in the first shell, 8 could fit in. First, find electrons of tin atom. Number of electrons in shells. Including scores of properties, element names in many languages,. 2nd shell can hold 8 electrons. In my textbook, it says that the. Tin Number Of Shell Electrons.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Tin Number Of Shell Electrons In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n². Comprehensive data on the chemical element tin is provided on this page; 4th shell can hold 32 electrons, and so on. The atomic number of tin represents the total number of electrons of tin. 3rd shell can hold. Tin Number Of Shell Electrons.
From www.nuclear-power.com
Tin Atomic Number Atomic Mass Density of Tin Tin Number Of Shell Electrons Thus, 1st shell can hold 2 electrons. 2nd shell can hold 8 electrons. Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. Comprehensive data on the chemical element tin is provided on this page; This would mean 2 electrons could fit in the first shell, 8 could fit in. The number of electrons in. Tin Number Of Shell Electrons.
From www.webelements.com
Elements Periodic Table » Tin » properties of free atoms Tin Number Of Shell Electrons Thus, 1st shell can hold 2 electrons. 4th shell can hold 32 electrons, and so on. Including scores of properties, element names in many languages,. Comprehensive data on the chemical element tin is provided on this page; First, find electrons of tin atom. In my textbook, it says that the maximum number of electrons that can fit in any given. Tin Number Of Shell Electrons.
From commons.wikimedia.org
FileElectron shell 050 tin.png Tin Number Of Shell Electrons Comprehensive data on the chemical element tin is provided on this page; In the periodic table, the elements are listed in order of increasing atomic number z. Including scores of properties, element names in many languages,. This would mean 2 electrons could fit in the first shell, 8 could fit in. The atomic number of tin represents the total number. Tin Number Of Shell Electrons.
From www.alamy.com
3d render of atom structure of tin isolated over white background Tin Number Of Shell Electrons Including scores of properties, element names in many languages,. Thus, 1st shell can hold 2 electrons. 2nd shell can hold 8 electrons. In the periodic table, the elements are listed in order of increasing atomic number z. Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. First, find electrons of tin atom. 3rd shell. Tin Number Of Shell Electrons.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 Tin Number Of Shell Electrons In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n². This would mean 2 electrons could fit in the first shell, 8 could fit in. Electron configuration of tin is [kr] 4d10 5s2 5p2. First, find electrons of tin atom. The atomic number of tin represents the total. Tin Number Of Shell Electrons.
From valenceelectrons.com
Tin(Sn) electron configuration and orbital diagram Tin Number Of Shell Electrons 3rd shell can hold 18 electrons. First, find electrons of tin atom. 2nd shell can hold 8 electrons. Including scores of properties, element names in many languages,. This would mean 2 electrons could fit in the first shell, 8 could fit in. The atomic number of tin represents the total number of electrons of tin. Comprehensive data on the chemical. Tin Number Of Shell Electrons.
From chemistryatomrevision.weebly.com
Electron Configuration Atomic Structure Tin Number Of Shell Electrons 2nd shell can hold 8 electrons. In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n². Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. The atomic number of tin represents the total number of electrons of tin. Number of electrons in. Tin Number Of Shell Electrons.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Tin Number Of Shell Electrons Thus, 1st shell can hold 2 electrons. Including scores of properties, element names in many languages,. Comprehensive data on the chemical element tin is provided on this page; 4th shell can hold 32 electrons, and so on. 3rd shell can hold 18 electrons. The atomic number of tin represents the total number of electrons of tin. In my textbook, it. Tin Number Of Shell Electrons.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Number Of Shell Electrons This would mean 2 electrons could fit in the first shell, 8 could fit in. Comprehensive data on the chemical element tin is provided on this page; Thus, 1st shell can hold 2 electrons. 4th shell can hold 32 electrons, and so on. Including scores of properties, element names in many languages,. 3rd shell can hold 18 electrons. First, find. Tin Number Of Shell Electrons.
From www.alamy.com
Periodic Table of the Elements shows atomic number, symbol, name Tin Number Of Shell Electrons In the periodic table, the elements are listed in order of increasing atomic number z. Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. First, find electrons of tin atom. This would mean 2 electrons could fit in the first shell, 8 could fit in. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. In. Tin Number Of Shell Electrons.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Number Of Shell Electrons Comprehensive data on the chemical element tin is provided on this page; 3rd shell can hold 18 electrons. Including scores of properties, element names in many languages,. First, find electrons of tin atom. This would mean 2 electrons could fit in the first shell, 8 could fit in. Tin’s electrons are distributed across five shells, following the 2, 8, 18,. Tin Number Of Shell Electrons.
From mungfali.com
Periodic Table Of Elements Showing Electron Shells Tin Number Of Shell Electrons Including scores of properties, element names in many languages,. 3rd shell can hold 18 electrons. In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n². The atomic number of tin represents the total number of electrons of tin. Electron configuration of tin is [kr] 4d10 5s2 5p2. Tin’s. Tin Number Of Shell Electrons.
From www.animalia-life.club
Electron Shell Diagram Tin Number Of Shell Electrons First, find electrons of tin atom. Comprehensive data on the chemical element tin is provided on this page; Electron configuration of tin is [kr] 4d10 5s2 5p2. Number of electrons in shells. In the periodic table, the elements are listed in order of increasing atomic number z. Including scores of properties, element names in many languages,. Thus, 1st shell can. Tin Number Of Shell Electrons.
From material-properties.org
Tin Protons Neutrons Electrons Electron Configuration Tin Number Of Shell Electrons In the periodic table, the elements are listed in order of increasing atomic number z. Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Sources, facts, uses, scarcity (sri), podcasts,. Tin Number Of Shell Electrons.