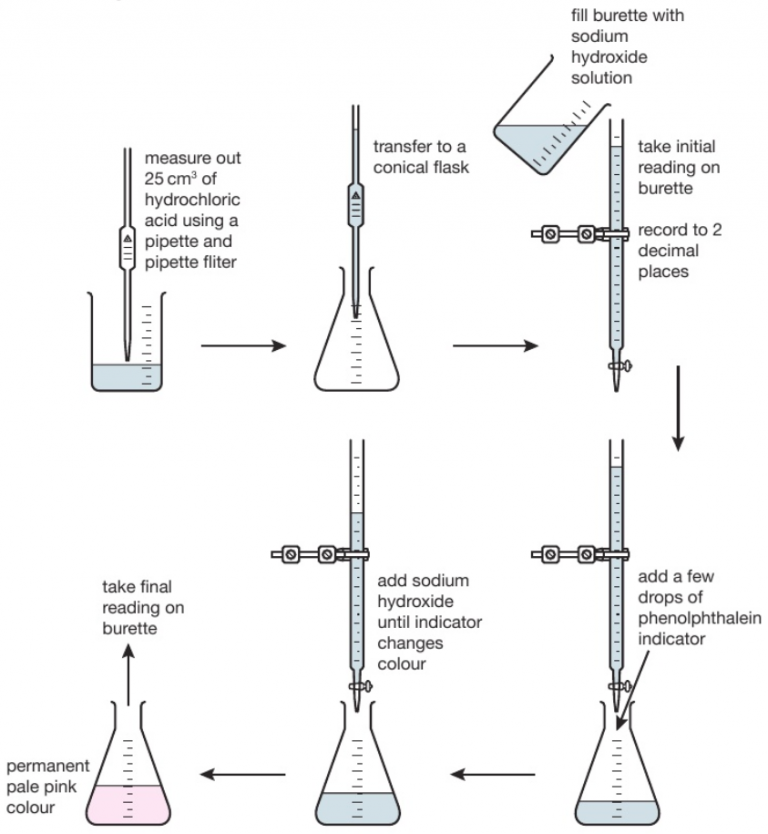
Titration Ka Chemistry . As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. This equilibrium constant is a quantitative measure of the strength of an acid in a solution. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. To know the relationship between acid or base strength and the magnitude of ka, kb, pka, and pkb. Calculate the concentration of the sodium hydroxide solution. H+ (aq) ion concentration and half equivalence point. There are tables of acid dissociation constants, for easy reference. K a is commonly expressed in units of mol/l. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. To understand the leveling effect. How to find the acid dissociation constant, ka, for an acid using a titration curve. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base.
from www.tutormyself.com
As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. To know the relationship between acid or base strength and the magnitude of ka, kb, pka, and pkb. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. Calculate the concentration of the sodium hydroxide solution. There are two basic types of acid base titrations, indicator and potentiometric. H+ (aq) ion concentration and half equivalence point. K a is commonly expressed in units of mol/l. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. To understand the leveling effect. There are tables of acid dissociation constants, for easy reference.
(f) Acids, alkalis and titrations Archives TutorMyself Chemistry
Titration Ka Chemistry The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. To understand the leveling effect. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. There are tables of acid dissociation constants, for easy reference. How to find the acid dissociation constant, ka, for an acid using a titration curve. H+ (aq) ion concentration and half equivalence point. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. K a is commonly expressed in units of mol/l. Calculate the concentration of the sodium hydroxide solution. This equilibrium constant is a quantitative measure of the strength of an acid in a solution. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. To know the relationship between acid or base strength and the magnitude of ka, kb, pka, and pkb.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Ka Chemistry How to find the acid dissociation constant, ka, for an acid using a titration curve. Calculate the concentration of the sodium hydroxide solution. There are tables of acid dissociation constants, for easy reference. To know the relationship between acid or base strength and the magnitude of ka, kb, pka, and pkb. At the equivalence point in a neutralization, the moles. Titration Ka Chemistry.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube Titration Ka Chemistry As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. K a is commonly expressed in units of mol/l. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. There are two basic types of acid. Titration Ka Chemistry.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Titration Ka Chemistry How to find the acid dissociation constant, ka, for an acid using a titration curve. K a is commonly expressed in units of mol/l. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. There are two basic types of acid base titrations, indicator and potentiometric. To know the. Titration Ka Chemistry.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Ka Chemistry There are two basic types of acid base titrations, indicator and potentiometric. To understand the leveling effect. How to find the acid dissociation constant, ka, for an acid using a titration curve. To know the relationship between acid or base strength and the magnitude of ka, kb, pka, and pkb. This equilibrium constant is a quantitative measure of the strength. Titration Ka Chemistry.
From chem.libretexts.org
AcidBase Titrations Chemistry LibreTexts Titration Ka Chemistry There are two basic types of acid base titrations, indicator and potentiometric. Calculate the concentration of the sodium hydroxide solution. H+ (aq) ion concentration and half equivalence point. To know the relationship between acid or base strength and the magnitude of ka, kb, pka, and pkb. To understand the leveling effect. K a is commonly expressed in units of mol/l.. Titration Ka Chemistry.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Ka Chemistry H+ (aq) ion concentration and half equivalence point. K a is commonly expressed in units of mol/l. Calculate the concentration of the sodium hydroxide solution. There are two basic types of acid base titrations, indicator and potentiometric. How to find the acid dissociation constant, ka, for an acid using a titration curve. This equilibrium constant is a quantitative measure of. Titration Ka Chemistry.
From www.youtube.com
Acid Base Titration Lecture 3 Chemistry Practicals Fsc 1st Year Titration Ka Chemistry At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. There are tables of acid dissociation constants, for easy reference. K a is commonly expressed in units of mol/l. To know the relationship between acid or base strength and the magnitude of ka, kb, pka, and pkb. There are two basic types. Titration Ka Chemistry.
From www.alamy.com
Titration apparatus at chemical laboratory Stock Photo 29817654 Alamy Titration Ka Chemistry There are two basic types of acid base titrations, indicator and potentiometric. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. This equilibrium constant is a quantitative measure of the. Titration Ka Chemistry.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Ka Chemistry How to find the acid dissociation constant, ka, for an acid using a titration curve. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. At the equivalence point in a neutralization, the moles of acid are equal to the moles. Titration Ka Chemistry.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Ka Chemistry At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. There are tables of acid dissociation constants, for easy reference. K a is commonly expressed in units of mol/l. To understand the leveling effect. Calculate the concentration of the sodium hydroxide solution. The acid dissociation constant is the equilibrium constant of the. Titration Ka Chemistry.
From www.alamy.com
Scientist performing titration in a chemistry laboratory with Redox Titration Ka Chemistry To understand the leveling effect. This equilibrium constant is a quantitative measure of the strength of an acid in a solution. There are two basic types of acid base titrations, indicator and potentiometric. H+ (aq) ion concentration and half equivalence point. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions. Titration Ka Chemistry.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Ka Chemistry As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. There are tables of acid dissociation constants, for easy reference. H+ (aq) ion concentration and half equivalence point. How to find the acid dissociation constant, ka, for an acid using a titration curve. There are two basic. Titration Ka Chemistry.
From facts.net
8 Captivating Facts About AcidBase Titration Titration Ka Chemistry At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. K a is commonly expressed in units of mol/l. There are tables of acid dissociation constants, for easy reference. Calculate the concentration of the sodium hydroxide solution. This equilibrium constant is a quantitative measure of the strength of an acid in a. Titration Ka Chemistry.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Ka Chemistry To understand the leveling effect. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. This equilibrium constant is a quantitative measure of the strength of an acid in a solution. To know the relationship between acid or base strength and the magnitude of ka, kb, pka,. Titration Ka Chemistry.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Titration Ka Chemistry At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. How to find the acid dissociation constant, ka, for an acid using a. Titration Ka Chemistry.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Ka Chemistry As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. H+ (aq) ion concentration and half equivalence point. How to find the acid dissociation constant, ka, for an acid using a titration curve. K a is commonly expressed in units of mol/l. At the equivalence point in. Titration Ka Chemistry.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Ka Chemistry The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. How to find the acid dissociation constant, ka, for an acid using a titration curve. There are two basic types of acid base titrations, indicator and potentiometric. There are tables of acid dissociation constants, for easy reference. To know. Titration Ka Chemistry.
From mungfali.com
Acid Base Titration Lab Titration Ka Chemistry H+ (aq) ion concentration and half equivalence point. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. How to find the acid dissociation constant, ka, for an acid using a titration curve. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. Titration Ka Chemistry.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Ka Chemistry H+ (aq) ion concentration and half equivalence point. There are two basic types of acid base titrations, indicator and potentiometric. K a is commonly expressed in units of mol/l. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. How to find the acid dissociation constant, ka, for an acid using a. Titration Ka Chemistry.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Ka Chemistry How to find the acid dissociation constant, ka, for an acid using a titration curve. To understand the leveling effect. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric. Titration Ka Chemistry.
From www.birmingham.ac.uk
Chemistry titrations University of Birmingham Titration Ka Chemistry There are tables of acid dissociation constants, for easy reference. Calculate the concentration of the sodium hydroxide solution. To understand the leveling effect. This equilibrium constant is a quantitative measure of the strength of an acid in a solution. To know the relationship between acid or base strength and the magnitude of ka, kb, pka, and pkb. At the equivalence. Titration Ka Chemistry.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Titration Ka Chemistry K a is commonly expressed in units of mol/l. There are tables of acid dissociation constants, for easy reference. Calculate the concentration of the sodium hydroxide solution. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. As seen in the chapter on the stoichiometry of chemical reactions, titrations. Titration Ka Chemistry.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Ka Chemistry To understand the leveling effect. Calculate the concentration of the sodium hydroxide solution. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. There are tables of acid dissociation constants, for easy reference. There are two basic types of acid base titrations, indicator and potentiometric. K a. Titration Ka Chemistry.
From www.tes.com
Titration Edexcel 91 Separate (Triple) Science Teaching Resources Titration Ka Chemistry To understand the leveling effect. How to find the acid dissociation constant, ka, for an acid using a titration curve. Calculate the concentration of the sodium hydroxide solution. H+ (aq) ion concentration and half equivalence point. There are tables of acid dissociation constants, for easy reference. This equilibrium constant is a quantitative measure of the strength of an acid in. Titration Ka Chemistry.
From fineartamerica.com
Acidbase Titration by Science Source Titration Ka Chemistry Calculate the concentration of the sodium hydroxide solution. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions.. Titration Ka Chemistry.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Ka Chemistry How to find the acid dissociation constant, ka, for an acid using a titration curve. To know the relationship between acid or base strength and the magnitude of ka, kb, pka, and pkb. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. This equilibrium constant is. Titration Ka Chemistry.
From www.chemicals.co.uk
What is Titration Used For in Real Life? The Chemistry Blog Titration Ka Chemistry To understand the leveling effect. This equilibrium constant is a quantitative measure of the strength of an acid in a solution. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. How to find the acid dissociation constant, ka, for an acid using a titration curve. There. Titration Ka Chemistry.
From www.scribd.com
Titration PDF Titration Chemistry Titration Ka Chemistry In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. How to find the acid dissociation constant, ka, for an acid using a. Titration Ka Chemistry.
From www.youtube.com
28 TITRATION 1 Experiment , Calculation HSC Chemistry YouTube Titration Ka Chemistry The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. Calculate the concentration of the sodium hydroxide solution. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence. Titration Ka Chemistry.
From www.tutormyself.com
(f) Acids, alkalis and titrations Archives TutorMyself Chemistry Titration Ka Chemistry At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. There are two basic types of acid base titrations, indicator and potentiometric. K a is commonly expressed in units of mol/l. To understand the leveling effect. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. Titration Ka Chemistry.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Ka Chemistry How to find the acid dissociation constant, ka, for an acid using a titration curve. There are two basic types of acid base titrations, indicator and potentiometric. There are tables of acid dissociation constants, for easy reference. H+ (aq) ion concentration and half equivalence point. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid. Titration Ka Chemistry.
From www.scribd.com
titration ( chemistry Experiment report) Titration Analysis Titration Ka Chemistry As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. How to find the acid dissociation constant, ka, for an acid using a titration curve. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. K a is commonly. Titration Ka Chemistry.
From www.youtube.com
Titration How to perform Titration Experiment/Practical YouTube Titration Ka Chemistry There are two basic types of acid base titrations, indicator and potentiometric. To know the relationship between acid or base strength and the magnitude of ka, kb, pka, and pkb. Calculate the concentration of the sodium hydroxide solution. There are tables of acid dissociation constants, for easy reference. To understand the leveling effect. How to find the acid dissociation constant,. Titration Ka Chemistry.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Ka Chemistry The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. There are tables of acid dissociation constants, for easy reference. This equilibrium constant is a quantitative measure of the strength of an acid in a solution. To know the relationship between acid or base strength and the magnitude of. Titration Ka Chemistry.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Titration Ka Chemistry As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. To understand the leveling effect. H+ (aq) ion concentration and half equivalence point. This equilibrium constant is a quantitative measure of the strength of an acid in a solution. There are tables of acid dissociation constants, for. Titration Ka Chemistry.