Chlorine Mechanism Reaction . A chlorine radical and a methyl radical can couple to. two chlorine radicals and couple to form more halogen reactant (cl 2). chlorination is the first reaction mechanism that you need to learn. Compounds other than chlorine and methane go through halogenation with the radical chain mechanism. Explain, in your own words, how the first propagation step can occur without input of energy if it is energetically unfavorable. write out the complete mechanism for the chlorination of methane. however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms. This mechanism involves free radicals. A free radical is a species with one (or more than one) unpaired electrons. the reaction pathway of methane chlorination can be modeled using a numerical approach.
from encyclopedia.pub
Explain, in your own words, how the first propagation step can occur without input of energy if it is energetically unfavorable. however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms. A chlorine radical and a methyl radical can couple to. two chlorine radicals and couple to form more halogen reactant (cl 2). This mechanism involves free radicals. write out the complete mechanism for the chlorination of methane. the reaction pathway of methane chlorination can be modeled using a numerical approach. A free radical is a species with one (or more than one) unpaired electrons. Compounds other than chlorine and methane go through halogenation with the radical chain mechanism. chlorination is the first reaction mechanism that you need to learn.
Fundamentals of Chlorine Disinfection Encyclopedia MDPI
Chlorine Mechanism Reaction Compounds other than chlorine and methane go through halogenation with the radical chain mechanism. A chlorine radical and a methyl radical can couple to. the reaction pathway of methane chlorination can be modeled using a numerical approach. This mechanism involves free radicals. write out the complete mechanism for the chlorination of methane. Explain, in your own words, how the first propagation step can occur without input of energy if it is energetically unfavorable. A free radical is a species with one (or more than one) unpaired electrons. however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms. chlorination is the first reaction mechanism that you need to learn. two chlorine radicals and couple to form more halogen reactant (cl 2). Compounds other than chlorine and methane go through halogenation with the radical chain mechanism.
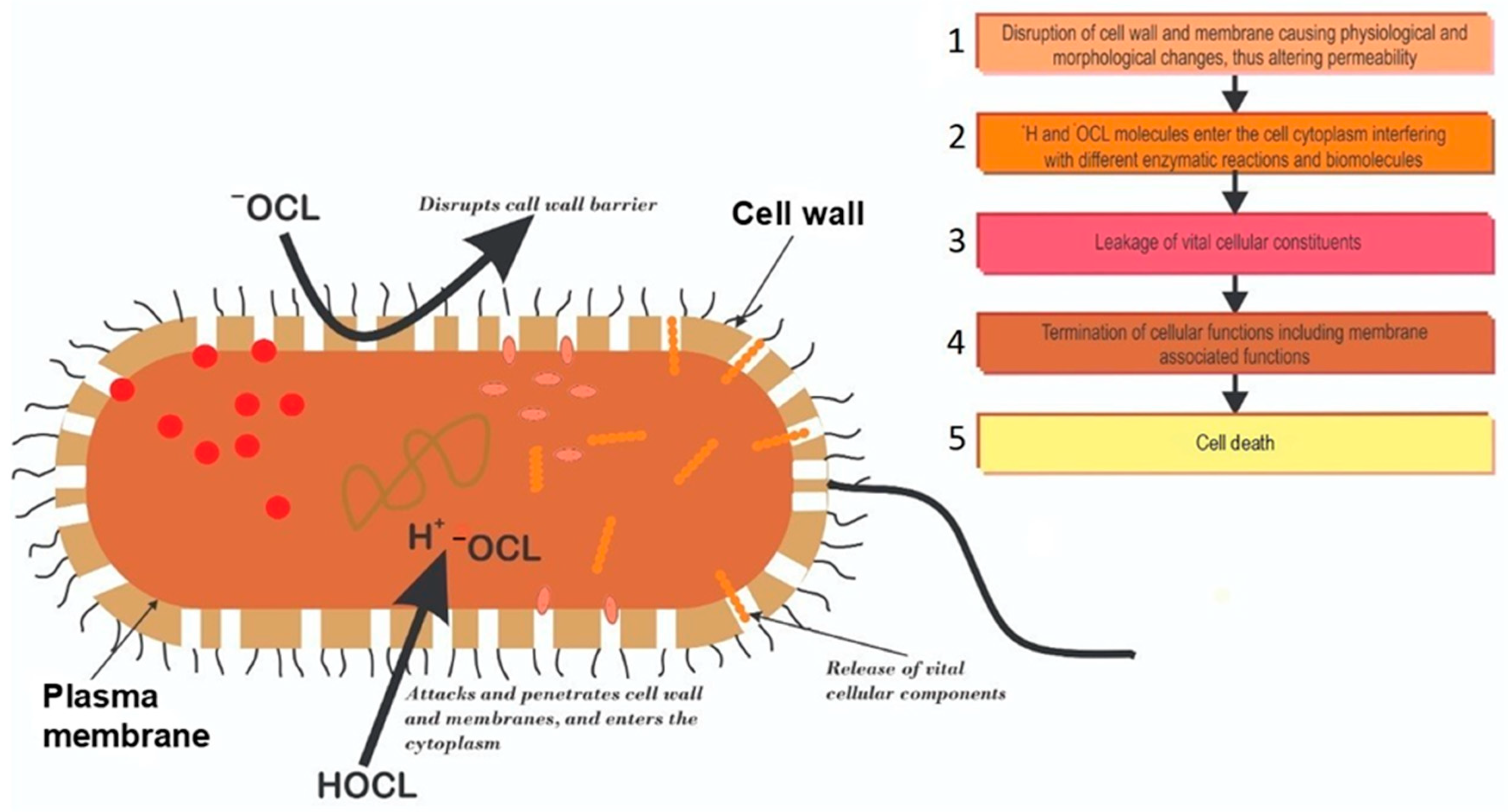
From www.cell.com
Reaction Direct chlorination of ethane to dichloroethane Chem Chlorine Mechanism Reaction Compounds other than chlorine and methane go through halogenation with the radical chain mechanism. the reaction pathway of methane chlorination can be modeled using a numerical approach. two chlorine radicals and couple to form more halogen reactant (cl 2). write out the complete mechanism for the chlorination of methane. however, the reaction doesn't stop there, and. Chlorine Mechanism Reaction.
From www.coursehero.com
[Solved] 11. Provide a stepwise curved arrow mechanism for the Chlorine Mechanism Reaction This mechanism involves free radicals. Compounds other than chlorine and methane go through halogenation with the radical chain mechanism. the reaction pathway of methane chlorination can be modeled using a numerical approach. A chlorine radical and a methyl radical can couple to. however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn. Chlorine Mechanism Reaction.
From www.researchgate.net
Important reaction pathways for DBP formation during chlorination and Chlorine Mechanism Reaction write out the complete mechanism for the chlorination of methane. A free radical is a species with one (or more than one) unpaired electrons. Explain, in your own words, how the first propagation step can occur without input of energy if it is energetically unfavorable. Compounds other than chlorine and methane go through halogenation with the radical chain mechanism.. Chlorine Mechanism Reaction.
From www.chemtube3d.com
A Level Radical Reactions Synthesis of Chloroalkanes Chlorine Mechanism Reaction This mechanism involves free radicals. chlorination is the first reaction mechanism that you need to learn. however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms. the reaction pathway of methane chlorination can be modeled using a numerical approach. A chlorine radical and a methyl radical. Chlorine Mechanism Reaction.
From mavink.com
Ethene And Chlorine Reaction Chlorine Mechanism Reaction This mechanism involves free radicals. chlorination is the first reaction mechanism that you need to learn. the reaction pathway of methane chlorination can be modeled using a numerical approach. two chlorine radicals and couple to form more halogen reactant (cl 2). Compounds other than chlorine and methane go through halogenation with the radical chain mechanism. however,. Chlorine Mechanism Reaction.
From www.britannica.com
Chemical reaction The BrønstedLowry theory Britannica Chlorine Mechanism Reaction chlorination is the first reaction mechanism that you need to learn. however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms. the reaction pathway of methane chlorination can be modeled using a numerical approach. A chlorine radical and a methyl radical can couple to. write. Chlorine Mechanism Reaction.
From www.researchgate.net
Scheme 2. Mechanism calculated of the chlorination reaction with Chlorine Mechanism Reaction A free radical is a species with one (or more than one) unpaired electrons. chlorination is the first reaction mechanism that you need to learn. Compounds other than chlorine and methane go through halogenation with the radical chain mechanism. A chlorine radical and a methyl radical can couple to. two chlorine radicals and couple to form more halogen. Chlorine Mechanism Reaction.
From ar.inspiredpencil.com
Electrophilic Aromatic Substitution Mechanism Chlorination Chlorine Mechanism Reaction Compounds other than chlorine and methane go through halogenation with the radical chain mechanism. A free radical is a species with one (or more than one) unpaired electrons. the reaction pathway of methane chlorination can be modeled using a numerical approach. however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be. Chlorine Mechanism Reaction.
From www.slideserve.com
PPT Reactions of chlorine with water and sodium hydroxide. PowerPoint Chlorine Mechanism Reaction This mechanism involves free radicals. A free radical is a species with one (or more than one) unpaired electrons. the reaction pathway of methane chlorination can be modeled using a numerical approach. A chlorine radical and a methyl radical can couple to. chlorination is the first reaction mechanism that you need to learn. Compounds other than chlorine and. Chlorine Mechanism Reaction.
From www.chegg.com
Solved Consider the mechanism shown below for chlorination Chlorine Mechanism Reaction chlorination is the first reaction mechanism that you need to learn. the reaction pathway of methane chlorination can be modeled using a numerical approach. A free radical is a species with one (or more than one) unpaired electrons. write out the complete mechanism for the chlorination of methane. This mechanism involves free radicals. however, the reaction. Chlorine Mechanism Reaction.
From www.numerade.com
SOLVED Chlorine and bromine react In the dark WIth alkenes The Chlorine Mechanism Reaction write out the complete mechanism for the chlorination of methane. A free radical is a species with one (or more than one) unpaired electrons. Explain, in your own words, how the first propagation step can occur without input of energy if it is energetically unfavorable. A chlorine radical and a methyl radical can couple to. Compounds other than chlorine. Chlorine Mechanism Reaction.
From www.mdpi.com
Catalysts Free FullText Chlorination of Toluene to oChlorotoluene Chlorine Mechanism Reaction A chlorine radical and a methyl radical can couple to. the reaction pathway of methane chlorination can be modeled using a numerical approach. chlorination is the first reaction mechanism that you need to learn. Compounds other than chlorine and methane go through halogenation with the radical chain mechanism. two chlorine radicals and couple to form more halogen. Chlorine Mechanism Reaction.
From www.youtube.com
Mechanism (Chlorination of Benzene ) YouTube Chlorine Mechanism Reaction two chlorine radicals and couple to form more halogen reactant (cl 2). the reaction pathway of methane chlorination can be modeled using a numerical approach. This mechanism involves free radicals. A free radical is a species with one (or more than one) unpaired electrons. write out the complete mechanism for the chlorination of methane. Compounds other than. Chlorine Mechanism Reaction.
From www.researchgate.net
Scheme 13 Reaction of chlorine dioxide with ketosulfides 4249 Chlorine Mechanism Reaction Compounds other than chlorine and methane go through halogenation with the radical chain mechanism. write out the complete mechanism for the chlorination of methane. This mechanism involves free radicals. however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms. Explain, in your own words, how the first. Chlorine Mechanism Reaction.
From www.researchgate.net
Simplified chemical reaction scheme of the generation of chlorine Chlorine Mechanism Reaction the reaction pathway of methane chlorination can be modeled using a numerical approach. Explain, in your own words, how the first propagation step can occur without input of energy if it is energetically unfavorable. A chlorine radical and a methyl radical can couple to. two chlorine radicals and couple to form more halogen reactant (cl 2). however,. Chlorine Mechanism Reaction.
From alevelchemistry.co.uk
Reactions of Alkanes ALevel Chemistry Revision Notes Chlorine Mechanism Reaction write out the complete mechanism for the chlorination of methane. This mechanism involves free radicals. A free radical is a species with one (or more than one) unpaired electrons. chlorination is the first reaction mechanism that you need to learn. Compounds other than chlorine and methane go through halogenation with the radical chain mechanism. the reaction pathway. Chlorine Mechanism Reaction.
From www.researchgate.net
Reaction mechanism for the chlorineradicalinitiated oxidation of Chlorine Mechanism Reaction chlorination is the first reaction mechanism that you need to learn. however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms. A chlorine radical and a methyl radical can couple to. This mechanism involves free radicals. two chlorine radicals and couple to form more halogen reactant. Chlorine Mechanism Reaction.
From www.toppr.com
In the free radical chlorination of methane, the c Chlorine Mechanism Reaction however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms. A chlorine radical and a methyl radical can couple to. chlorination is the first reaction mechanism that you need to learn. This mechanism involves free radicals. two chlorine radicals and couple to form more halogen reactant. Chlorine Mechanism Reaction.
From www.masterorganicchemistry.com
Thionyl Chloride (SOCl2) Master Organic Chemistry Chlorine Mechanism Reaction however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms. Explain, in your own words, how the first propagation step can occur without input of energy if it is energetically unfavorable. This mechanism involves free radicals. A chlorine radical and a methyl radical can couple to. write. Chlorine Mechanism Reaction.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes — Master Organic Chemistry Chlorine Mechanism Reaction the reaction pathway of methane chlorination can be modeled using a numerical approach. This mechanism involves free radicals. write out the complete mechanism for the chlorination of methane. A chlorine radical and a methyl radical can couple to. A free radical is a species with one (or more than one) unpaired electrons. chlorination is the first reaction. Chlorine Mechanism Reaction.
From quizlet.com
Chlorination of Methane Diagram Quizlet Chlorine Mechanism Reaction write out the complete mechanism for the chlorination of methane. A free radical is a species with one (or more than one) unpaired electrons. however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms. chlorination is the first reaction mechanism that you need to learn. This. Chlorine Mechanism Reaction.
From www.numerade.com
SOLVED What is the first step in the curved arrow mechanism for the Chlorine Mechanism Reaction chlorination is the first reaction mechanism that you need to learn. however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms. two chlorine radicals and couple to form more halogen reactant (cl 2). This mechanism involves free radicals. A chlorine radical and a methyl radical can. Chlorine Mechanism Reaction.
From www.essentialchemicalindustry.org
Chlorine Chlorine Mechanism Reaction A free radical is a species with one (or more than one) unpaired electrons. Explain, in your own words, how the first propagation step can occur without input of energy if it is energetically unfavorable. write out the complete mechanism for the chlorination of methane. chlorination is the first reaction mechanism that you need to learn. the. Chlorine Mechanism Reaction.
From www.researchgate.net
Mechanism of chlorination treatment in MAFC. Download Scientific Diagram Chlorine Mechanism Reaction however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms. the reaction pathway of methane chlorination can be modeled using a numerical approach. chlorination is the first reaction mechanism that you need to learn. two chlorine radicals and couple to form more halogen reactant (cl. Chlorine Mechanism Reaction.
From www.researchgate.net
Scheme I. Reactions of chlorine with a fragment of residual lignin Chlorine Mechanism Reaction two chlorine radicals and couple to form more halogen reactant (cl 2). the reaction pathway of methane chlorination can be modeled using a numerical approach. A free radical is a species with one (or more than one) unpaired electrons. chlorination is the first reaction mechanism that you need to learn. write out the complete mechanism for. Chlorine Mechanism Reaction.
From www.chemistryscl.com
Benzene and Chlorine Reaction C6H6 + Cl2, Mechanism Chlorine Mechanism Reaction A free radical is a species with one (or more than one) unpaired electrons. A chlorine radical and a methyl radical can couple to. two chlorine radicals and couple to form more halogen reactant (cl 2). write out the complete mechanism for the chlorination of methane. chlorination is the first reaction mechanism that you need to learn.. Chlorine Mechanism Reaction.
From www.chemistryscl.com
propagation of chains in methane chlorination Chlorine Mechanism Reaction write out the complete mechanism for the chlorination of methane. the reaction pathway of methane chlorination can be modeled using a numerical approach. A chlorine radical and a methyl radical can couple to. This mechanism involves free radicals. A free radical is a species with one (or more than one) unpaired electrons. chlorination is the first reaction. Chlorine Mechanism Reaction.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Chain reaction Chlorine Mechanism Reaction This mechanism involves free radicals. the reaction pathway of methane chlorination can be modeled using a numerical approach. write out the complete mechanism for the chlorination of methane. chlorination is the first reaction mechanism that you need to learn. A chlorine radical and a methyl radical can couple to. A free radical is a species with one. Chlorine Mechanism Reaction.
From www.doubtnut.com
Discuss the reaction mechanism of following reactions (1) Chlorine Mechanism Reaction A free radical is a species with one (or more than one) unpaired electrons. Explain, in your own words, how the first propagation step can occur without input of energy if it is energetically unfavorable. A chlorine radical and a methyl radical can couple to. the reaction pathway of methane chlorination can be modeled using a numerical approach. Compounds. Chlorine Mechanism Reaction.
From byjus.com
In the free radical chlorination of methane, the chain initiation step Chlorine Mechanism Reaction the reaction pathway of methane chlorination can be modeled using a numerical approach. Compounds other than chlorine and methane go through halogenation with the radical chain mechanism. however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms. Explain, in your own words, how the first propagation step. Chlorine Mechanism Reaction.
From encyclopedia.pub
Fundamentals of Chlorine Disinfection Encyclopedia MDPI Chlorine Mechanism Reaction This mechanism involves free radicals. Compounds other than chlorine and methane go through halogenation with the radical chain mechanism. Explain, in your own words, how the first propagation step can occur without input of energy if it is energetically unfavorable. chlorination is the first reaction mechanism that you need to learn. A free radical is a species with one. Chlorine Mechanism Reaction.
From www.researchgate.net
4. Mechanism of chlorine atom (Cl) release responsible for ozone (O 3 Chlorine Mechanism Reaction write out the complete mechanism for the chlorination of methane. the reaction pathway of methane chlorination can be modeled using a numerical approach. This mechanism involves free radicals. two chlorine radicals and couple to form more halogen reactant (cl 2). however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn. Chlorine Mechanism Reaction.
From pubs.acs.org
Chlorination of Aliphatic Primary Alcohols via Triphosgene Chlorine Mechanism Reaction two chlorine radicals and couple to form more halogen reactant (cl 2). write out the complete mechanism for the chlorination of methane. Compounds other than chlorine and methane go through halogenation with the radical chain mechanism. however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms.. Chlorine Mechanism Reaction.
From chemtube3d.com
Radicals Chlorination of alkanes Chlorine Mechanism Reaction A free radical is a species with one (or more than one) unpaired electrons. This mechanism involves free radicals. A chlorine radical and a methyl radical can couple to. however, the reaction doesn't stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms. two chlorine radicals and couple to form more. Chlorine Mechanism Reaction.
From www.researchgate.net
Energetically diagram of the pathways of the reaction chlorine dioxide Chlorine Mechanism Reaction Compounds other than chlorine and methane go through halogenation with the radical chain mechanism. write out the complete mechanism for the chlorination of methane. chlorination is the first reaction mechanism that you need to learn. This mechanism involves free radicals. the reaction pathway of methane chlorination can be modeled using a numerical approach. A chlorine radical and. Chlorine Mechanism Reaction.