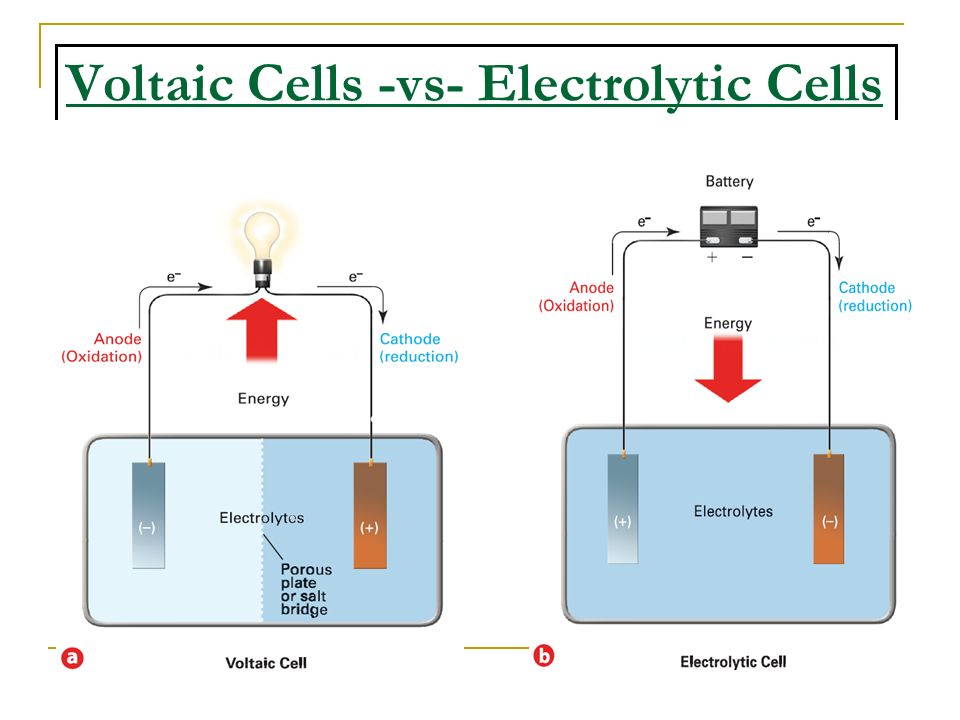
Function Of Electrode In Galvanic Cell . electrochemical cells allow measurement and control of a redox reaction. By definition, the anode of an electrochemical cell is the electrode at which oxidation. The name refers to the flow of anions in. The reaction can be started and stopped by connecting or disconnecting the two. Left to right in the standard galvanic cell in the figure. electrons flow from the anode to the cathode: Electrolyte is an insulator for electrons, but a conductor for the oxidized/reduced species migrating from anode/cathode to cathode/anode, which keeps the charge neutrality in the cell. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil).
from www.reddit.com
Electrolyte is an insulator for electrons, but a conductor for the oxidized/reduced species migrating from anode/cathode to cathode/anode, which keeps the charge neutrality in the cell. electrochemical cells allow measurement and control of a redox reaction. electrons flow from the anode to the cathode: The name refers to the flow of anions in. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). Left to right in the standard galvanic cell in the figure. The reaction can be started and stopped by connecting or disconnecting the two. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). By definition, the anode of an electrochemical cell is the electrode at which oxidation.
AAMC Physics QPack 27 Electron Movement r/Mcat
Function Of Electrode In Galvanic Cell by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). Electrolyte is an insulator for electrons, but a conductor for the oxidized/reduced species migrating from anode/cathode to cathode/anode, which keeps the charge neutrality in the cell. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). By definition, the anode of an electrochemical cell is the electrode at which oxidation. Left to right in the standard galvanic cell in the figure. electrochemical cells allow measurement and control of a redox reaction. electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). The name refers to the flow of anions in. The reaction can be started and stopped by connecting or disconnecting the two.
From socratic.org
How can a galvanic cell an electrolytic cell? Socratic Function Of Electrode In Galvanic Cell Left to right in the standard galvanic cell in the figure. The name refers to the flow of anions in. electrochemical cells allow measurement and control of a redox reaction. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where. Function Of Electrode In Galvanic Cell.
From chem.libretexts.org
Chapter 19.6 Corrosion Chemistry LibreTexts Function Of Electrode In Galvanic Cell The name refers to the flow of anions in. The reaction can be started and stopped by connecting or disconnecting the two. electrochemical cells allow measurement and control of a redox reaction. Left to right in the standard galvanic cell in the figure. electrons flow from the anode to the cathode: Electrolyte is an insulator for electrons, but. Function Of Electrode In Galvanic Cell.
From www.vrogue.co
Chapter 3 Electrochemistry Galvanic Cell Part 1 Youtu vrogue.co Function Of Electrode In Galvanic Cell electrochemical cells allow measurement and control of a redox reaction. By definition, the anode of an electrochemical cell is the electrode at which oxidation. The name refers to the flow of anions in. Left to right in the standard galvanic cell in the figure. by definition, the anode of an electrochemical cell is the electrode at which oxidation. Function Of Electrode In Galvanic Cell.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts Function Of Electrode In Galvanic Cell electrochemical cells allow measurement and control of a redox reaction. Electrolyte is an insulator for electrons, but a conductor for the oxidized/reduced species migrating from anode/cathode to cathode/anode, which keeps the charge neutrality in the cell. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and. Function Of Electrode In Galvanic Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Function Of Electrode In Galvanic Cell electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). Left to right in the standard galvanic cell in the figure. The name refers to the flow. Function Of Electrode In Galvanic Cell.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts Function Of Electrode In Galvanic Cell Left to right in the standard galvanic cell in the figure. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). The name refers to the flow of anions in. By definition, the anode of an. Function Of Electrode In Galvanic Cell.
From www.researchgate.net
The convention three electrode cell configuration for electrochemical Function Of Electrode In Galvanic Cell The name refers to the flow of anions in. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). By definition, the anode of an electrochemical cell is the electrode at which oxidation. electrons flow. Function Of Electrode In Galvanic Cell.
From semcouniversity.com
How the three electrode system works Semco University Semco Function Of Electrode In Galvanic Cell electrons flow from the anode to the cathode: electrochemical cells allow measurement and control of a redox reaction. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). Left to right in the standard. Function Of Electrode In Galvanic Cell.
From www.pinterest.com
What is Galvanic Cell? in 2020 Galvanic cell, Cell, Electronic products Function Of Electrode In Galvanic Cell The name refers to the flow of anions in. Left to right in the standard galvanic cell in the figure. electrochemical cells allow measurement and control of a redox reaction. electrons flow from the anode to the cathode: By definition, the anode of an electrochemical cell is the electrode at which oxidation. by definition, the anode of. Function Of Electrode In Galvanic Cell.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Function Of Electrode In Galvanic Cell Left to right in the standard galvanic cell in the figure. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). electrons flow from the anode to the cathode: Electrolyte is an insulator for electrons,. Function Of Electrode In Galvanic Cell.
From nigerianscholars.com
Electrochemistry Tutorials Nigerian Scholars Function Of Electrode In Galvanic Cell The reaction can be started and stopped by connecting or disconnecting the two. Left to right in the standard galvanic cell in the figure. Electrolyte is an insulator for electrons, but a conductor for the oxidized/reduced species migrating from anode/cathode to cathode/anode, which keeps the charge neutrality in the cell. by definition, the anode of an electrochemical cell is. Function Of Electrode In Galvanic Cell.
From 9to5science.com
[Solved] Why does electron flow from anode? 9to5Science Function Of Electrode In Galvanic Cell By definition, the anode of an electrochemical cell is the electrode at which oxidation. electrochemical cells allow measurement and control of a redox reaction. electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is. Function Of Electrode In Galvanic Cell.
From www.vrogue.co
Galvanic Cell Animation Galvanic Cell Cell Electrical vrogue.co Function Of Electrode In Galvanic Cell electrons flow from the anode to the cathode: The name refers to the flow of anions in. By definition, the anode of an electrochemical cell is the electrode at which oxidation. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. Function Of Electrode In Galvanic Cell.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Function Of Electrode In Galvanic Cell by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). Left to right in the standard galvanic cell in the figure. The name refers to the flow of anions in. Electrolyte is an insulator for electrons,. Function Of Electrode In Galvanic Cell.
From psu.pb.unizin.org
17.3 Standard Reduction Potentials Chemistry 112 Chapters 1217 of Function Of Electrode In Galvanic Cell electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). The name refers to the flow of anions in. by definition, the anode of an electrochemical. Function Of Electrode In Galvanic Cell.
From fjelloghjem.blogspot.com
18 Fresh Piston Engine Diagram Function Of Electrode In Galvanic Cell Electrolyte is an insulator for electrons, but a conductor for the oxidized/reduced species migrating from anode/cathode to cathode/anode, which keeps the charge neutrality in the cell. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil).. Function Of Electrode In Galvanic Cell.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation Function Of Electrode In Galvanic Cell by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). electrons flow from the anode to the cathode: The reaction can be started and stopped by connecting or disconnecting the two. Left to right in. Function Of Electrode In Galvanic Cell.
From www.pinterest.co.uk
What are the differences between Galvanic cell and Electrolytic cell Function Of Electrode In Galvanic Cell The name refers to the flow of anions in. By definition, the anode of an electrochemical cell is the electrode at which oxidation. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). Electrolyte is an. Function Of Electrode In Galvanic Cell.
From psu.pb.unizin.org
Galvanic Cells (17.2) Chemistry 110 Function Of Electrode In Galvanic Cell by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). Electrolyte is an insulator for electrons, but a conductor for the oxidized/reduced species migrating from anode/cathode to cathode/anode, which keeps the charge neutrality in the cell.. Function Of Electrode In Galvanic Cell.
From chemistry.stackexchange.com
electrochemistry Standard electrode potential for electrolytic vs Function Of Electrode In Galvanic Cell Left to right in the standard galvanic cell in the figure. electrochemical cells allow measurement and control of a redox reaction. The name refers to the flow of anions in. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where. Function Of Electrode In Galvanic Cell.
From courses.lumenlearning.com
Electrolysis Chemistry Function Of Electrode In Galvanic Cell By definition, the anode of an electrochemical cell is the electrode at which oxidation. electrochemical cells allow measurement and control of a redox reaction. The reaction can be started and stopped by connecting or disconnecting the two. Electrolyte is an insulator for electrons, but a conductor for the oxidized/reduced species migrating from anode/cathode to cathode/anode, which keeps the charge. Function Of Electrode In Galvanic Cell.
From www.researchgate.net
17. A schematic view of electrochemical setup 3electrode cell in a Function Of Electrode In Galvanic Cell The name refers to the flow of anions in. By definition, the anode of an electrochemical cell is the electrode at which oxidation. The reaction can be started and stopped by connecting or disconnecting the two. Left to right in the standard galvanic cell in the figure. by definition, the anode of an electrochemical cell is the electrode at. Function Of Electrode In Galvanic Cell.
From www.slideserve.com
PPT Electrochemistry Part II The Galvanic Cell PowerPoint Function Of Electrode In Galvanic Cell The reaction can be started and stopped by connecting or disconnecting the two. The name refers to the flow of anions in. electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where. Function Of Electrode In Galvanic Cell.
From gambr.co
️Labeling Electrochemical Cell Diagram Worksheet Free Download Gambr.co Function Of Electrode In Galvanic Cell Left to right in the standard galvanic cell in the figure. electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). The name refers to the flow. Function Of Electrode In Galvanic Cell.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Function Of Electrode In Galvanic Cell by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). The reaction can be started and stopped by connecting or disconnecting the two. Left to right in the standard galvanic cell in the figure. Electrolyte is. Function Of Electrode In Galvanic Cell.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID5404902 Function Of Electrode In Galvanic Cell Electrolyte is an insulator for electrons, but a conductor for the oxidized/reduced species migrating from anode/cathode to cathode/anode, which keeps the charge neutrality in the cell. electrons flow from the anode to the cathode: Left to right in the standard galvanic cell in the figure. The name refers to the flow of anions in. The reaction can be started. Function Of Electrode In Galvanic Cell.
From ar.inspiredpencil.com
Galvanic Cell Labeled Function Of Electrode In Galvanic Cell Electrolyte is an insulator for electrons, but a conductor for the oxidized/reduced species migrating from anode/cathode to cathode/anode, which keeps the charge neutrality in the cell. electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode. Function Of Electrode In Galvanic Cell.
From users.highland.edu
Commercial Galvanic Cells Function Of Electrode In Galvanic Cell The name refers to the flow of anions in. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). electrochemical cells allow measurement and control of a redox reaction. Left to right in the standard. Function Of Electrode In Galvanic Cell.
From thechemistrynotes.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle Function Of Electrode In Galvanic Cell by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). Left to right in the standard galvanic cell in the figure. The name refers to the flow of anions in. by definition, the anode of. Function Of Electrode In Galvanic Cell.
From www.differencebetween.com
Difference Between Galvanic Cell and Concentration Cell Compare the Function Of Electrode In Galvanic Cell electrochemical cells allow measurement and control of a redox reaction. By definition, the anode of an electrochemical cell is the electrode at which oxidation. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). Left. Function Of Electrode In Galvanic Cell.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download Function Of Electrode In Galvanic Cell The reaction can be started and stopped by connecting or disconnecting the two. electrons flow from the anode to the cathode: by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). Left to right in. Function Of Electrode In Galvanic Cell.
From www.reddit.com
AAMC Physics QPack 27 Electron Movement r/Mcat Function Of Electrode In Galvanic Cell By definition, the anode of an electrochemical cell is the electrode at which oxidation. The name refers to the flow of anions in. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). by definition,. Function Of Electrode In Galvanic Cell.
From www.chegg.com
Solved A galvanic cell is made up of a copper electrode in a Function Of Electrode In Galvanic Cell electrochemical cells allow measurement and control of a redox reaction. By definition, the anode of an electrochemical cell is the electrode at which oxidation. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). Electrolyte. Function Of Electrode In Galvanic Cell.
From ar.inspiredpencil.com
Galvanic Cell Function Of Electrode In Galvanic Cell The reaction can be started and stopped by connecting or disconnecting the two. electrochemical cells allow measurement and control of a redox reaction. electrons flow from the anode to the cathode: Electrolyte is an insulator for electrons, but a conductor for the oxidized/reduced species migrating from anode/cathode to cathode/anode, which keeps the charge neutrality in the cell. . Function Of Electrode In Galvanic Cell.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Function Of Electrode In Galvanic Cell by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). electrons flow from the anode to the cathode: By definition, the anode of an electrochemical cell is the electrode at which oxidation. The name refers. Function Of Electrode In Galvanic Cell.