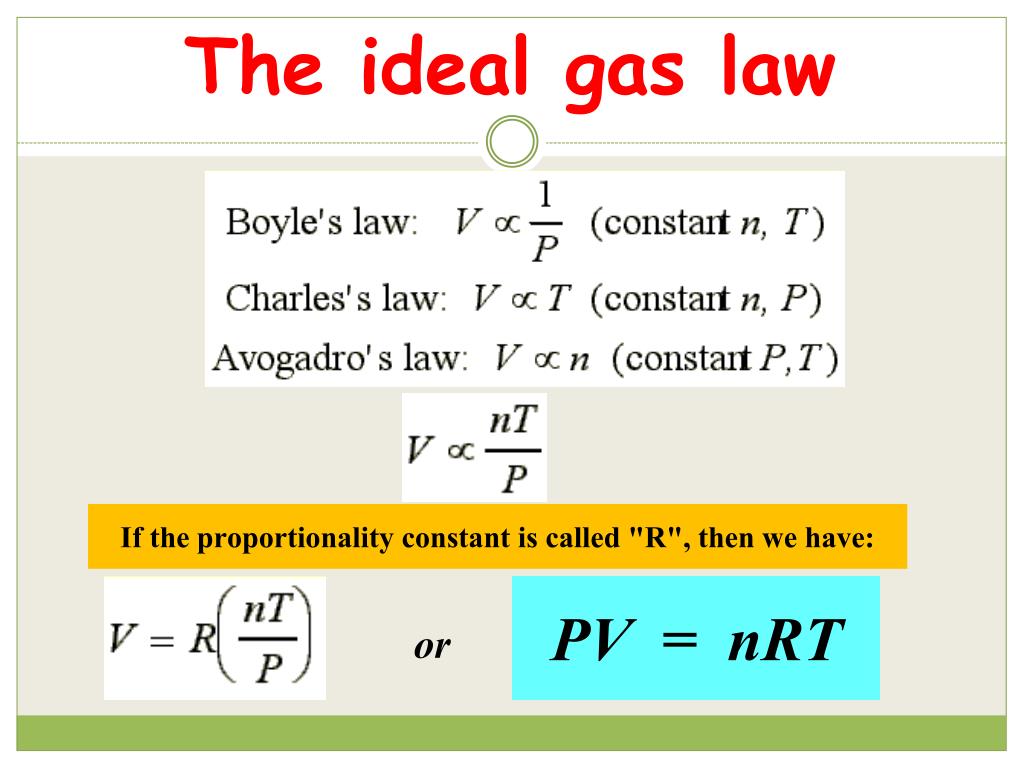
What Does R Mean In Ideal Gas Law . the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so learning this one means that you have learned them all. p v = n r t. Properties of the gaseous state predicted by the ideal gas law are within 5% for gases under ordinary conditions. definition of an ideal gas, ideal gas law (video) | khan academy. Where p is the pressure of the gas, v is the volume taken up by the gas, t is the temperature of the gas, r is the gas constant, and n is the number of. The simple gas laws can always be. where r is known as the gas constant, is called the ideal gas law or equation of state. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases.
from www.slideserve.com
Properties of the gaseous state predicted by the ideal gas law are within 5% for gases under ordinary conditions. where r is known as the gas constant, is called the ideal gas law or equation of state. p v = n r t. definition of an ideal gas, ideal gas law (video) | khan academy. Where p is the pressure of the gas, v is the volume taken up by the gas, t is the temperature of the gas, r is the gas constant, and n is the number of. The simple gas laws can always be. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so learning this one means that you have learned them all.
PPT Gases PowerPoint Presentation, free download ID4387987
What Does R Mean In Ideal Gas Law definition of an ideal gas, ideal gas law (video) | khan academy. the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so learning this one means that you have learned them all. definition of an ideal gas, ideal gas law (video) | khan academy. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. The simple gas laws can always be. Where p is the pressure of the gas, v is the volume taken up by the gas, t is the temperature of the gas, r is the gas constant, and n is the number of. where r is known as the gas constant, is called the ideal gas law or equation of state. p v = n r t. Properties of the gaseous state predicted by the ideal gas law are within 5% for gases under ordinary conditions.
From cavenger.blogspot.com
Ideal Gas Law R Values Gas Stoichiometry The Ideal Gas Law Science What Does R Mean In Ideal Gas Law the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so learning this one means that you have learned them all. Where p is the pressure of the gas, v is the volume taken up by the gas, t is the temperature of the gas, r is the. What Does R Mean In Ideal Gas Law.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net What Does R Mean In Ideal Gas Law the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so learning this one means. What Does R Mean In Ideal Gas Law.
From www.slideserve.com
PPT THE PROPERTIES OF GASES PowerPoint Presentation, free download What Does R Mean In Ideal Gas Law where r is known as the gas constant, is called the ideal gas law or equation of state. the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so learning this one means that you have learned them all. the ideal gas law describes the behavior. What Does R Mean In Ideal Gas Law.
From erebusryling.blogspot.com
Ideal Gas Law R Values PPT Gas Laws PowerPoint Presentation ID What Does R Mean In Ideal Gas Law The simple gas laws can always be. p v = n r t. Where p is the pressure of the gas, v is the volume taken up by the gas, t is the temperature of the gas, r is the gas constant, and n is the number of. the ideal gas law describes the behavior of an ideal. What Does R Mean In Ideal Gas Law.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID What Does R Mean In Ideal Gas Law Where p is the pressure of the gas, v is the volume taken up by the gas, t is the temperature of the gas, r is the gas constant, and n is the number of. the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so learning this. What Does R Mean In Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 What Does R Mean In Ideal Gas Law Properties of the gaseous state predicted by the ideal gas law are within 5% for gases under ordinary conditions. where r is known as the gas constant, is called the ideal gas law or equation of state. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by. What Does R Mean In Ideal Gas Law.
From poornima-kulkarni.blogspot.com
Ideal Gas Law R Values Solved The Ideal Gas Law Is Given By Pv = RT What Does R Mean In Ideal Gas Law Where p is the pressure of the gas, v is the volume taken up by the gas, t is the temperature of the gas, r is the gas constant, and n is the number of. Properties of the gaseous state predicted by the ideal gas law are within 5% for gases under ordinary conditions. the ideal gas law describes. What Does R Mean In Ideal Gas Law.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID7067134 What Does R Mean In Ideal Gas Law the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. Where p is the pressure of the gas, v is the volume taken up by the gas, t is the temperature of the gas, r is the. What Does R Mean In Ideal Gas Law.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation What Does R Mean In Ideal Gas Law the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. where r is known as the gas constant, is called the ideal gas law or equation of state. Where p is the pressure of the gas,. What Does R Mean In Ideal Gas Law.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID6624513 What Does R Mean In Ideal Gas Law the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. definition of an ideal gas, ideal gas law (video) | khan academy. where r is known as the gas constant, is called the ideal gas. What Does R Mean In Ideal Gas Law.
From www.youtube.com
Introduction to the Ideal Gas Law Formula YouTube What Does R Mean In Ideal Gas Law Where p is the pressure of the gas, v is the volume taken up by the gas, t is the temperature of the gas, r is the gas constant, and n is the number of. definition of an ideal gas, ideal gas law (video) | khan academy. Properties of the gaseous state predicted by the ideal gas law are. What Does R Mean In Ideal Gas Law.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii What Does R Mean In Ideal Gas Law p v = n r t. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's. What Does R Mean In Ideal Gas Law.
From studylib.net
IdealGas Equation The constant of proportionality is R What Does R Mean In Ideal Gas Law the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. Properties of the gaseous state predicted by the ideal gas law are within 5% for gases under ordinary conditions. where r is known as the gas. What Does R Mean In Ideal Gas Law.
From www.youtube.com
Ideal Gas Law YouTube What Does R Mean In Ideal Gas Law the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. Where p is the pressure of the gas, v is the volume taken up by the gas, t is the temperature of the gas, r is the. What Does R Mean In Ideal Gas Law.
From stock.adobe.com
PV = nRT Ideal Gas Law Brings Together Gas Properties. The Most What Does R Mean In Ideal Gas Law The simple gas laws can always be. definition of an ideal gas, ideal gas law (video) | khan academy. Properties of the gaseous state predicted by the ideal gas law are within 5% for gases under ordinary conditions. where r is known as the gas constant, is called the ideal gas law or equation of state. the. What Does R Mean In Ideal Gas Law.
From bbc-morning-news-update29.blogspot.com
Ideal Gas Law R Values 5.4 The Ideal Gas Law YouTube What Does R Mean In Ideal Gas Law p v = n r t. the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so learning this one means that you have learned them all. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained. What Does R Mean In Ideal Gas Law.
From www.chem.fsu.edu
Gas Laws What Does R Mean In Ideal Gas Law p v = n r t. the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so learning this one means that you have learned them all. definition of an ideal gas, ideal gas law (video) | khan academy. Where p is the pressure of the. What Does R Mean In Ideal Gas Law.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID3344204 What Does R Mean In Ideal Gas Law p v = n r t. the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so learning this one means that you have learned them all. definition of an ideal gas, ideal gas law (video) | khan academy. Where p is the pressure of the. What Does R Mean In Ideal Gas Law.
From sciencenotes.org
Ideal Gas Law Formula and Examples What Does R Mean In Ideal Gas Law The simple gas laws can always be. definition of an ideal gas, ideal gas law (video) | khan academy. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. p v = n r t.. What Does R Mean In Ideal Gas Law.
From nokmanuk8.blogspot.com
R Values Ideal Gas Law Bar PPT Ideal Gas Law PowerPoint What Does R Mean In Ideal Gas Law p v = n r t. the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so learning this one means that you have learned them all. definition of an ideal gas, ideal gas law (video) | khan academy. where r is known as the. What Does R Mean In Ideal Gas Law.
From www.slideserve.com
PPT The ideal gas law PowerPoint Presentation, free download ID3608170 What Does R Mean In Ideal Gas Law where r is known as the gas constant, is called the ideal gas law or equation of state. Where p is the pressure of the gas, v is the volume taken up by the gas, t is the temperature of the gas, r is the gas constant, and n is the number of. the ideal gas law describes. What Does R Mean In Ideal Gas Law.
From www.slideserve.com
PPT Chapter 5 Gases PowerPoint Presentation, free download ID593425 What Does R Mean In Ideal Gas Law the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. Where p is the pressure of the gas, v is the volume taken up by the gas, t is the temperature of the gas, r is the. What Does R Mean In Ideal Gas Law.
From www.slideshare.net
Ideal gas law practice mccpot What Does R Mean In Ideal Gas Law the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so learning this one means that you have learned them all. definition of an ideal gas, ideal gas law (video) | khan academy. Where p is the pressure of the gas, v is the volume taken up. What Does R Mean In Ideal Gas Law.
From byjus.com
Ideal Gas Law Equation Compressibility Of Natural Gas Chemistry What Does R Mean In Ideal Gas Law definition of an ideal gas, ideal gas law (video) | khan academy. Properties of the gaseous state predicted by the ideal gas law are within 5% for gases under ordinary conditions. where r is known as the gas constant, is called the ideal gas law or equation of state. the ideal gas law describes the behavior of. What Does R Mean In Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law 01 PowerPoint Presentation, free download ID What Does R Mean In Ideal Gas Law p v = n r t. Properties of the gaseous state predicted by the ideal gas law are within 5% for gases under ordinary conditions. Where p is the pressure of the gas, v is the volume taken up by the gas, t is the temperature of the gas, r is the gas constant, and n is the number. What Does R Mean In Ideal Gas Law.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4387987 What Does R Mean In Ideal Gas Law p v = n r t. definition of an ideal gas, ideal gas law (video) | khan academy. Properties of the gaseous state predicted by the ideal gas law are within 5% for gases under ordinary conditions. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. What Does R Mean In Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID5758540 What Does R Mean In Ideal Gas Law the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. definition of an ideal gas, ideal gas law (video) | khan academy. Where p is the pressure of the gas, v is the volume taken up. What Does R Mean In Ideal Gas Law.
From www.youtube.com
Ideal Gas Equation How to Choose the Correct Gas Constant, R? With What Does R Mean In Ideal Gas Law p v = n r t. definition of an ideal gas, ideal gas law (video) | khan academy. Properties of the gaseous state predicted by the ideal gas law are within 5% for gases under ordinary conditions. the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law),. What Does R Mean In Ideal Gas Law.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii What Does R Mean In Ideal Gas Law the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. Where p is the pressure of the gas, v is the volume taken up by the gas, t is the temperature of the gas, r is the. What Does R Mean In Ideal Gas Law.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii What Does R Mean In Ideal Gas Law where r is known as the gas constant, is called the ideal gas law or equation of state. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. the ideal gas law is simply the. What Does R Mean In Ideal Gas Law.
From mmerevise.co.uk
The Ideal Gas Equation MME What Does R Mean In Ideal Gas Law p v = n r t. the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so learning this one means that you have learned them all. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained. What Does R Mean In Ideal Gas Law.
From erebusryling.blogspot.com
Ideal Gas Law R Values PPT Gas Laws PowerPoint Presentation ID What Does R Mean In Ideal Gas Law the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. where r is known as the gas constant, is called the ideal gas law or equation of state. definition of an ideal gas, ideal gas. What Does R Mean In Ideal Gas Law.
From www.youtube.com
Ideal Gas Constant R EQUATION CHEMISTRY PV=nRT class 11 1st year What Does R Mean In Ideal Gas Law definition of an ideal gas, ideal gas law (video) | khan academy. the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so learning this one means that you have learned them all. Where p is the pressure of the gas, v is the volume taken up. What Does R Mean In Ideal Gas Law.
From bbc-morning-news-update29.blogspot.com
Ideal Gas Law R Values 5.4 The Ideal Gas Law YouTube What Does R Mean In Ideal Gas Law p v = n r t. Properties of the gaseous state predicted by the ideal gas law are within 5% for gases under ordinary conditions. definition of an ideal gas, ideal gas law (video) | khan academy. the ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law),. What Does R Mean In Ideal Gas Law.
From gaspinsami.blogspot.com
Gas Ideal Gas Law What Does R Mean In Ideal Gas Law the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. Where p is the pressure of the gas, v is the volume taken up by the gas, t is the temperature of the gas, r is the. What Does R Mean In Ideal Gas Law.