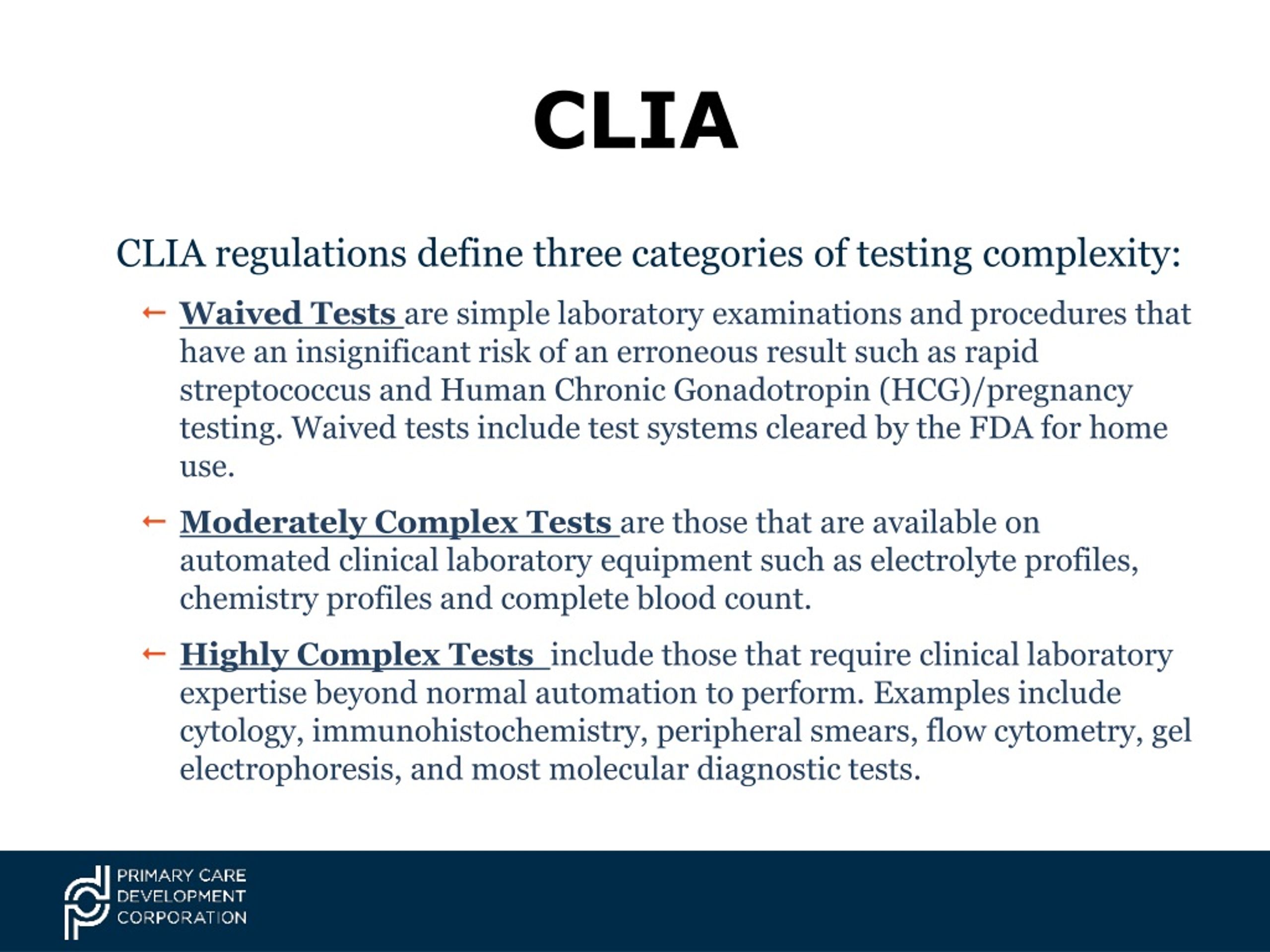
Clia Centrifuge Regulations . This information is on the centers for disease. Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. The most current version of the clia regulations part 493, including all changes through 5/12/14. Responded to public comments, cliac recommendations. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003.
from www.slideserve.com
The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. Responded to public comments, cliac recommendations. The most current version of the clia regulations part 493, including all changes through 5/12/14. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. This information is on the centers for disease.
PPT Step by Step Initiating and/or Enhancing Billable Services
Clia Centrifuge Regulations Responded to public comments, cliac recommendations. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. Responded to public comments, cliac recommendations. The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. This information is on the centers for disease. The most current version of the clia regulations part 493, including all changes through 5/12/14.
From www.scribd.com
CLIA Brochure 2 Regulations PDF Accuracy And Precision Reference Clia Centrifuge Regulations Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. The most current version of the clia regulations part 493, including all changes through 5/12/14. This information is on the centers for disease. Responded to public comments, cliac recommendations. The dual 510(k) and clia waiver application (dual submission), in which. Clia Centrifuge Regulations.
From www.bioagilytix.com
CLIA, COLA & CAP What’s the Difference? Navigating Regulations Clia Centrifuge Regulations The most current version of the clia regulations part 493, including all changes through 5/12/14. This information is on the centers for disease. The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. This clia compliance manual contains policies and procedures to help you comply with the final. Clia Centrifuge Regulations.
From klaffqmlf.blob.core.windows.net
Determine The Best Position For The Lid Of The Centrifuge During Each Clia Centrifuge Regulations The most current version of the clia regulations part 493, including all changes through 5/12/14. The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. This information is on the centers for disease. This clia compliance manual contains policies and procedures to help you comply with the final. Clia Centrifuge Regulations.
From www.mybiosource.com
Ensuring Quality and Accuracy in Laboratory Testing An Overview of Clia Centrifuge Regulations The most current version of the clia regulations part 493, including all changes through 5/12/14. This information is on the centers for disease. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. Responded to public comments, cliac recommendations. The dual 510(k) and clia. Clia Centrifuge Regulations.
From www.forermedchina.com
Operating Regulations of Low Speed Centrifuge Blog Henan Forever Clia Centrifuge Regulations The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement. Clia Centrifuge Regulations.
From www.slideserve.com
PPT Method Validation Studies It’s a Jungle out There! PowerPoint Clia Centrifuge Regulations Responded to public comments, cliac recommendations. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. The most current version of the clia regulations part 493, including all changes through 5/12/14. This information is on the centers for disease. The dual 510(k) and clia. Clia Centrifuge Regulations.
From www.collaborativetherapeutics.com
CLIA Application Video Tutorial + Checklist Clia Centrifuge Regulations Responded to public comments, cliac recommendations. The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. This information is on the centers. Clia Centrifuge Regulations.
From www.stuvia.com
CLIA Regulations and Lab management questions and answers 2024 with Clia Centrifuge Regulations The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. The most current version of the clia regulations part 493, including all changes through 5/12/14. This information is on the centers for disease. This clia compliance manual contains policies and procedures to help you comply with the final. Clia Centrifuge Regulations.
From shop.alfalaval.com
Decanter centrifuges Alfa Laval US Clia Centrifuge Regulations This information is on the centers for disease. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. Responded to public comments, cliac recommendations. The most current version of the clia regulations part 493, including all changes through 5/12/14. Understanding the regulations and standards. Clia Centrifuge Regulations.
From dokumen.tips
(PDF) ISSUES IN BRIEF CLIA INSPECTION CHECKLIST …€¦ · CLIA INSPECTION Clia Centrifuge Regulations This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. Responded to public comments, cliac recommendations. The dual 510(k) and clia waiver application (dual. Clia Centrifuge Regulations.
From www.prolisphere.com
What is CLIA Certification and How do I get CLIA certification? Clia Centrifuge Regulations The most current version of the clia regulations part 493, including all changes through 5/12/14. Responded to public comments, cliac recommendations. The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. This information is on the centers for disease. This clia compliance manual contains policies and procedures to. Clia Centrifuge Regulations.
From www.slideshare.net
Clia final regulations Clia Centrifuge Regulations The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. This information is on the centers for disease. The most current version of the clia regulations part 493,. Clia Centrifuge Regulations.
From studylib.net
Lab Regulations MI CLIA Policy Clia Centrifuge Regulations This information is on the centers for disease. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. The most current version of the. Clia Centrifuge Regulations.
From www.needle.tube
Understanding Clia Regulations Clia Centrifuge Regulations The most current version of the clia regulations part 493, including all changes through 5/12/14. This information is on the centers for disease. The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is. Clia Centrifuge Regulations.
From www.thermofisher.com
Working with CLIA Lab Regulations LIMS System Clia Centrifuge Regulations Responded to public comments, cliac recommendations. The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. This information is on the centers for disease. The most current version of the clia regulations part 493, including all changes through 5/12/14. Understanding the regulations and standards for selecting a centrifuge. Clia Centrifuge Regulations.
From www.clingengenomics.com
CLIA Laboratory Regulations site Clia Centrifuge Regulations Responded to public comments, cliac recommendations. This information is on the centers for disease. The most current version of the clia regulations part 493, including all changes through 5/12/14. Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. The dual 510(k) and clia waiver application (dual submission), in which. Clia Centrifuge Regulations.
From www.thermofisher.com
DynaSpin SingleUse Centrifuge Thermo Fisher Scientific US Clia Centrifuge Regulations Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. The most current version of the clia regulations part 493, including all changes through. Clia Centrifuge Regulations.
From www.separatech.com
3phase Decanter Centrifuge for Fish Oil Processing Clia Centrifuge Regulations This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. This information is on the centers for disease. The most current version. Clia Centrifuge Regulations.
From www.medicalequipment-msl.com
Chemiluminescence Immunoassay (CLIA) System machine Maglumi600 Clia Centrifuge Regulations Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. The most current version of the clia regulations part 493, including all changes through. Clia Centrifuge Regulations.
From www.slideserve.com
PPT Step by Step Initiating and/or Enhancing Billable Services Clia Centrifuge Regulations Responded to public comments, cliac recommendations. The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. This information is on the centers for disease. The most current version of the clia regulations part 493, including all changes through 5/12/14. Understanding the regulations and standards for selecting a centrifuge. Clia Centrifuge Regulations.
From ntep.in
Biosafety Requirements in the LPA Lab Refrigerated Centrifuge Use Clia Centrifuge Regulations This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. Responded to public comments, cliac recommendations. The most current version of the. Clia Centrifuge Regulations.
From www.youtube.com
Understanding CLIA and CAP Regulations to Advance Your Laboratory Clia Centrifuge Regulations Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. The most current version of the clia regulations part 493, including all changes through. Clia Centrifuge Regulations.
From krewinstruments.com
Cooling Centrifuge Krew Instruments Pvt Ltd Clia Centrifuge Regulations This information is on the centers for disease. The most current version of the clia regulations part 493, including all changes through 5/12/14. Responded to public comments, cliac recommendations. Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. The dual 510(k) and clia waiver application (dual submission), in which. Clia Centrifuge Regulations.
From iqlabconsultants.com
Ensuring Compliance In Your Lab CLIA Regulations Demystified Lab Clia Centrifuge Regulations Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement. Clia Centrifuge Regulations.
From www.slideserve.com
PPT LRI Validation Suite Meeting PowerPoint Presentation, free Clia Centrifuge Regulations The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. Understanding the regulations and standards for selecting a centrifuge in a medical. Clia Centrifuge Regulations.
From www.mlo-online.com
CLIA and regulatory readiness How can your lab always be ready? Clia Centrifuge Regulations The most current version of the clia regulations part 493, including all changes through 5/12/14. Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24,. Clia Centrifuge Regulations.
From slidetodoc.com
Paradise Senior Health Center A HospitalBased Clinic of Clia Centrifuge Regulations The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. Responded to public comments, cliac recommendations. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. This information is on the centers. Clia Centrifuge Regulations.
From www.lighthouselabservices.com
What Is a CLIA Lab Director and What Are Their Requirements? Clia Centrifuge Regulations The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. The most current version of the clia regulations part 493, including all changes through 5/12/14. Responded to public. Clia Centrifuge Regulations.
From arup.utah.edu
ARUP Scientific Resource for Research and Education MLS Resource Clia Centrifuge Regulations This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. This information is on the centers for disease. Understanding the regulations and. Clia Centrifuge Regulations.
From www.slideserve.com
PPT Complying With CLIA Competency Requirements PowerPoint Clia Centrifuge Regulations This information is on the centers for disease. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. Responded to public comments, cliac recommendations.. Clia Centrifuge Regulations.
From www.slideserve.com
PPT Clinical Laboratory Improvement Amendments of 1988 ( CLIA ’ 88 Clia Centrifuge Regulations The most current version of the clia regulations part 493, including all changes through 5/12/14. This information is on the centers for disease. Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. Responded to public comments, cliac recommendations. The dual 510(k) and clia waiver application (dual submission), in which. Clia Centrifuge Regulations.
From www.scribd.com
CLIA Regulations Pertaining To Your Lab Quality Assurance Food And Clia Centrifuge Regulations The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. The most current version of the clia regulations part 493, including all. Clia Centrifuge Regulations.
From app.livestorm.co
Changes to CLIA 2024 — Opportunities to Optimize Lab Quality and Reduce Clia Centrifuge Regulations This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. Understanding the regulations and standards for selecting a centrifuge in a medical diagnostic lab is crucial for ensuring quality and. The most current version of the clia regulations part 493, including all changes through. Clia Centrifuge Regulations.
From microbiomedicals.com
Laboratory/Micro Centrifuges MICRO Enterprises Medical Clia Centrifuge Regulations This information is on the centers for disease. This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia waiver concurrently. The most current version. Clia Centrifuge Regulations.
From www.boekelsci.com
Economy Centrifuges From Boekel Shop Now Clia Centrifuge Regulations This clia compliance manual contains policies and procedures to help you comply with the final clinical laboratory improvement amendments, which became effective on april 24, 2003. Responded to public comments, cliac recommendations. This information is on the centers for disease. The dual 510(k) and clia waiver application (dual submission), in which an applicant can apply for 510(k) clearance and clia. Clia Centrifuge Regulations.