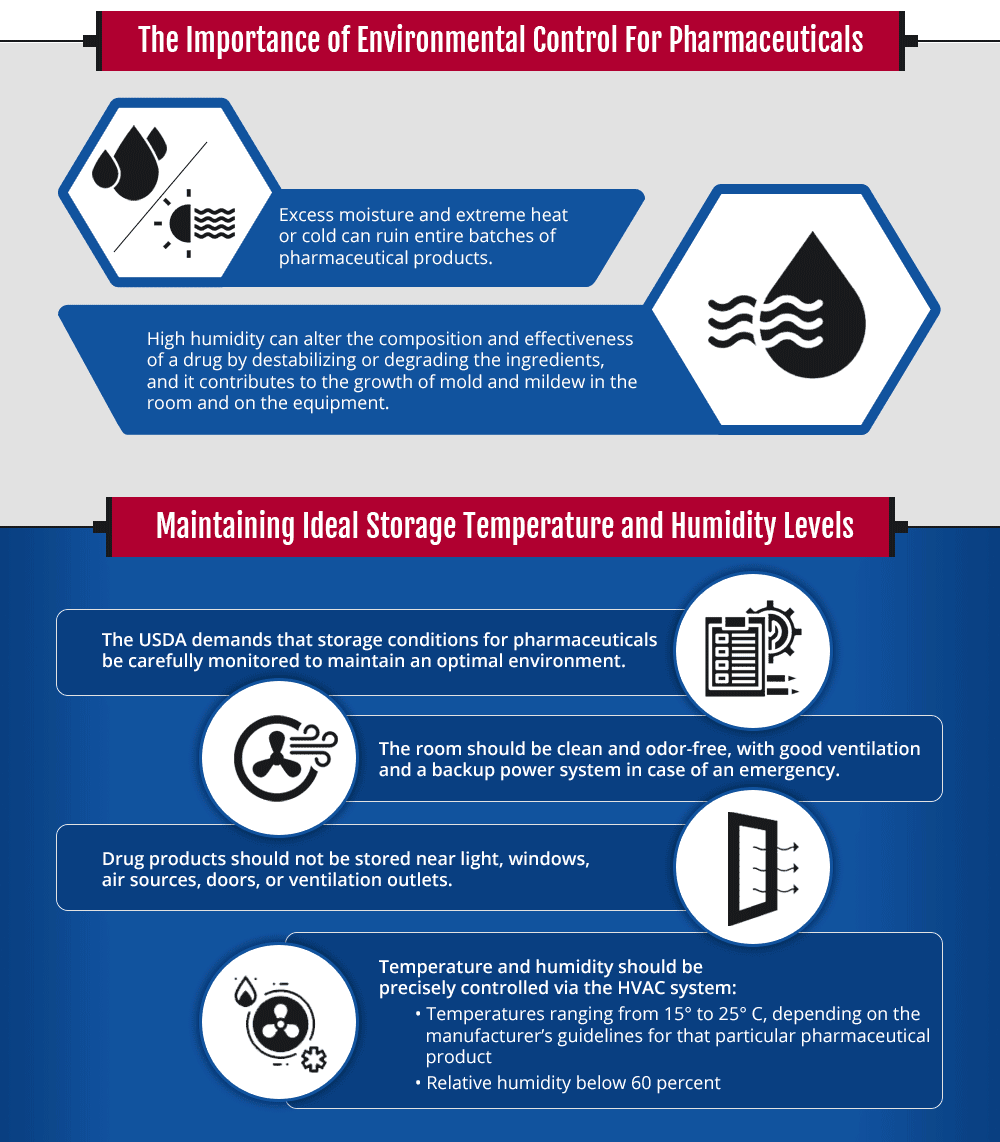
Temperature Storage Conditions For Pharmaceuticals . For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing. In particular, they should be clean and dry and maintained within acceptable temperature limits. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. 4.3 storage areas should be designed or adapted to ensure good storage conditions. Find entries and build and print. In particular, they should be clean and dry and maintained. Storage areas should be designed or adapted to ensure good storage conditions.
from airinnovations.com
In particular, they should be clean and dry and maintained. Find entries and build and print. 4.3 storage areas should be designed or adapted to ensure good storage conditions. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing. In particular, they should be clean and dry and maintained within acceptable temperature limits. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. Storage areas should be designed or adapted to ensure good storage conditions.
Pharmaceutical Temperature & Humidity Control Solutions Air Innovations
Temperature Storage Conditions For Pharmaceuticals 4.3 storage areas should be designed or adapted to ensure good storage conditions. Storage areas should be designed or adapted to ensure good storage conditions. In particular, they should be clean and dry and maintained within acceptable temperature limits. 4.3 storage areas should be designed or adapted to ensure good storage conditions. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. Find entries and build and print. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing. In particular, they should be clean and dry and maintained.
From klaezdvfj.blob.core.windows.net
Drug Storage Temperature Guidelines at Dustin Hubbell blog Temperature Storage Conditions For Pharmaceuticals In particular, they should be clean and dry and maintained within acceptable temperature limits. 4.3 storage areas should be designed or adapted to ensure good storage conditions. Storage areas should be designed or adapted to ensure good storage conditions. Find entries and build and print. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements. Temperature Storage Conditions For Pharmaceuticals.
From alithadnews.com
Pharmaceutical Temperature Controlled Storage Alit Had News Get Temperature Storage Conditions For Pharmaceuticals In particular, they should be clean and dry and maintained within acceptable temperature limits. 4.3 storage areas should be designed or adapted to ensure good storage conditions. Storage areas should be designed or adapted to ensure good storage conditions. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage. Temperature Storage Conditions For Pharmaceuticals.
From www.akcp.com
Temperature Excursion Management in Pharmaceutical Storage Temperature Storage Conditions For Pharmaceuticals In particular, they should be clean and dry and maintained. 4.3 storage areas should be designed or adapted to ensure good storage conditions. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing. In particular, they should be clean and dry and maintained within acceptable temperature limits.. Temperature Storage Conditions For Pharmaceuticals.
From manoxblog.com
Introduction to Temperature Mapping of Controlled Temperature Storage Temperature Storage Conditions For Pharmaceuticals In particular, they should be clean and dry and maintained within acceptable temperature limits. Find entries and build and print. Storage areas should be designed or adapted to ensure good storage conditions. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. For the purposes of this document, ‘controlled. Temperature Storage Conditions For Pharmaceuticals.
From edwards-transport.com
Temperature Controlled Storage Warehouse Facilities Edwards Transport Temperature Storage Conditions For Pharmaceuticals Storage areas should be designed or adapted to ensure good storage conditions. Find entries and build and print. 4.3 storage areas should be designed or adapted to ensure good storage conditions. In particular, they should be clean and dry and maintained. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring. Temperature Storage Conditions For Pharmaceuticals.
From prescriptionhope.com
Medication Storage, Where, How, Temperatures, Types, and Guidelines Temperature Storage Conditions For Pharmaceuticals In particular, they should be clean and dry and maintained within acceptable temperature limits. 4.3 storage areas should be designed or adapted to ensure good storage conditions. Find entries and build and print. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing. This document aims to. Temperature Storage Conditions For Pharmaceuticals.
From www.akcp.com
Monitoring Pharmaceutical Storage Temperature AKCP Solutions Temperature Storage Conditions For Pharmaceuticals This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. Storage areas should be designed or adapted to ensure good storage conditions. In particular, they should be clean and dry and maintained. Find entries and build and print. 4.3 storage areas should be designed or adapted to ensure good. Temperature Storage Conditions For Pharmaceuticals.
From www.researchgate.net
Storage temperature conditions Download Scientific Diagram Temperature Storage Conditions For Pharmaceuticals Find entries and build and print. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. In particular, they should be clean and dry and maintained. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing.. Temperature Storage Conditions For Pharmaceuticals.
From joisgrbdc.blob.core.windows.net
Storage Temperature Range For Medical Devices at Myrtle Holland blog Temperature Storage Conditions For Pharmaceuticals In particular, they should be clean and dry and maintained. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing. 4.3 storage areas should be designed. Temperature Storage Conditions For Pharmaceuticals.
From joisgrbdc.blob.core.windows.net
Storage Temperature Range For Medical Devices at Myrtle Holland blog Temperature Storage Conditions For Pharmaceuticals For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing. Storage areas should be designed or adapted to ensure good storage conditions. In particular, they should be clean and dry and maintained within acceptable temperature limits. 4.3 storage areas should be designed or adapted to ensure good. Temperature Storage Conditions For Pharmaceuticals.
From www.hsssearch.co.uk
HSS Controlled temperature storage the role of automation Temperature Storage Conditions For Pharmaceuticals For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. In particular, they should be clean and dry and maintained. 4.3 storage areas should be designed. Temperature Storage Conditions For Pharmaceuticals.
From manoxblog.com
Introduction to Temperature Mapping of Controlled Temperature Storage Temperature Storage Conditions For Pharmaceuticals Storage areas should be designed or adapted to ensure good storage conditions. Find entries and build and print. In particular, they should be clean and dry and maintained. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. 4.3 storage areas should be designed or adapted to ensure good. Temperature Storage Conditions For Pharmaceuticals.
From www.academia.edu
(PDF) Roomtemperature storage of medications labeled for refrigeration Temperature Storage Conditions For Pharmaceuticals Find entries and build and print. In particular, they should be clean and dry and maintained. 4.3 storage areas should be designed or adapted to ensure good storage conditions. In particular, they should be clean and dry and maintained within acceptable temperature limits. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of. Temperature Storage Conditions For Pharmaceuticals.
From q1scientific.com
ICH Quality Guidelines for Pharmaceutical Stability Storage Q1 Scientific Temperature Storage Conditions For Pharmaceuticals Storage areas should be designed or adapted to ensure good storage conditions. 4.3 storage areas should be designed or adapted to ensure good storage conditions. In particular, they should be clean and dry and maintained. In particular, they should be clean and dry and maintained within acceptable temperature limits. This document aims to set out uniform statements on storage conditions. Temperature Storage Conditions For Pharmaceuticals.
From temperaturecontrolledcontainerhire.co.uk
Storing Pharmaceuticals in Temperature Controlled Containers Temperature Storage Conditions For Pharmaceuticals In particular, they should be clean and dry and maintained within acceptable temperature limits. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing. Storage areas should be designed or adapted to ensure good storage conditions. Find entries and build and print. 4.3 storage areas should be. Temperature Storage Conditions For Pharmaceuticals.
From edwards-transport.com
Temperature Controlled Storage Warehouse Facilities Edwards Transport Temperature Storage Conditions For Pharmaceuticals In particular, they should be clean and dry and maintained. In particular, they should be clean and dry and maintained within acceptable temperature limits. Storage areas should be designed or adapted to ensure good storage conditions. Find entries and build and print. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not. Temperature Storage Conditions For Pharmaceuticals.
From www.biobanking.com
UltraLow Temperature Storage of Tissues and Blood Specimens Temperature Storage Conditions For Pharmaceuticals Find entries and build and print. In particular, they should be clean and dry and maintained within acceptable temperature limits. 4.3 storage areas should be designed or adapted to ensure good storage conditions. In particular, they should be clean and dry and maintained. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products. Temperature Storage Conditions For Pharmaceuticals.
From temperaturecontrolledcontainerhire.co.uk
Storing Pharmaceuticals in Temperature Controlled Containers Temperature Storage Conditions For Pharmaceuticals For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing. Find entries and build and print. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. Storage areas should be designed or adapted to ensure good. Temperature Storage Conditions For Pharmaceuticals.
From www.ijbti.com
Accurate temperature representation of storage conditions of human Temperature Storage Conditions For Pharmaceuticals 4.3 storage areas should be designed or adapted to ensure good storage conditions. Find entries and build and print. Storage areas should be designed or adapted to ensure good storage conditions. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing. In particular, they should be clean. Temperature Storage Conditions For Pharmaceuticals.
From www.monnit.com
Laboratory and Pharmaceutical Temperature Monitoring Systems Temperature Storage Conditions For Pharmaceuticals 4.3 storage areas should be designed or adapted to ensure good storage conditions. Storage areas should be designed or adapted to ensure good storage conditions. Find entries and build and print. In particular, they should be clean and dry and maintained. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products. Temperature Storage Conditions For Pharmaceuticals.
From www.pharmalogisticsiq.com
The Quality and Monitoring of Temperature Conditions for Storage and Temperature Storage Conditions For Pharmaceuticals This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. 4.3 storage areas should be designed or adapted to ensure good storage conditions. In particular, they should be clean and dry and maintained within acceptable temperature limits. In particular, they should be clean and dry and maintained. For the. Temperature Storage Conditions For Pharmaceuticals.
From www.grainger.com
AMERICAN BIOTECH SUPPLY Temperature Controlled Room Room Temp, 25°C Temperature Storage Conditions For Pharmaceuticals 4.3 storage areas should be designed or adapted to ensure good storage conditions. In particular, they should be clean and dry and maintained. In particular, they should be clean and dry and maintained within acceptable temperature limits. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. Find entries. Temperature Storage Conditions For Pharmaceuticals.
From airinnovations.com
Pharmaceutical Temperature & Humidity Control Solutions Air Innovations Temperature Storage Conditions For Pharmaceuticals In particular, they should be clean and dry and maintained. 4.3 storage areas should be designed or adapted to ensure good storage conditions. Find entries and build and print. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing. This document aims to set out uniform statements. Temperature Storage Conditions For Pharmaceuticals.
From www.crspharmasolutions.ie
Pharmaceutical Temperature Ranges CRS Pharma Temperature Storage Conditions For Pharmaceuticals For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing. Storage areas should be designed or adapted to ensure good storage conditions. 4.3 storage areas should be designed or adapted to ensure good storage conditions. In particular, they should be clean and dry and maintained. This document. Temperature Storage Conditions For Pharmaceuticals.
From www.akcp.com
Monitoring Pharmaceutical Storage Temperature AKCP Solutions Temperature Storage Conditions For Pharmaceuticals In particular, they should be clean and dry and maintained within acceptable temperature limits. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. Find entries and build and print. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring. Temperature Storage Conditions For Pharmaceuticals.
From www.emergency-live.com
Cómo almacenar medicamentos en la naturaleza y el entorno EMS ¡lea las Temperature Storage Conditions For Pharmaceuticals This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. In particular, they should be clean and dry and maintained within acceptable temperature limits. In particular, they should be clean and dry and maintained. 4.3 storage areas should be designed or adapted to ensure good storage conditions. Find entries. Temperature Storage Conditions For Pharmaceuticals.
From www.akcp.com
Monitoring Pharmaceutical Storage Temperature AKCP Solutions Temperature Storage Conditions For Pharmaceuticals 4.3 storage areas should be designed or adapted to ensure good storage conditions. In particular, they should be clean and dry and maintained. Storage areas should be designed or adapted to ensure good storage conditions. In particular, they should be clean and dry and maintained within acceptable temperature limits. Find entries and build and print. This document aims to set. Temperature Storage Conditions For Pharmaceuticals.
From www.researchgate.net
Storage performances for temperaturesensitive pharmaceuticals at Temperature Storage Conditions For Pharmaceuticals This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. In particular, they should be clean and dry and maintained within acceptable temperature limits. In particular, they should be clean and dry and maintained. Storage areas should be designed or adapted to ensure good storage conditions. 4.3 storage areas. Temperature Storage Conditions For Pharmaceuticals.
From trumedsystems.com
COVID19 Vaccine Temperature Stability Chart TruMed Systems Temperature Storage Conditions For Pharmaceuticals Storage areas should be designed or adapted to ensure good storage conditions. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. 4.3 storage areas should. Temperature Storage Conditions For Pharmaceuticals.
From wms.org
TemperatureStability Temperature Storage Conditions For Pharmaceuticals Find entries and build and print. 4.3 storage areas should be designed or adapted to ensure good storage conditions. In particular, they should be clean and dry and maintained within acceptable temperature limits. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring cold storage or freezing. This document aims to. Temperature Storage Conditions For Pharmaceuticals.
From www.intestthermal.com
Why Temperature Regulation is Important When Storing Pharmaceuticals Temperature Storage Conditions For Pharmaceuticals Storage areas should be designed or adapted to ensure good storage conditions. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. In particular, they should be clean and dry and maintained. In particular, they should be clean and dry and maintained within acceptable temperature limits. For the purposes. Temperature Storage Conditions For Pharmaceuticals.
From www.etcprg.cz
Temperature mapping of cooling boxes for the transport of Temperature Storage Conditions For Pharmaceuticals 4.3 storage areas should be designed or adapted to ensure good storage conditions. Storage areas should be designed or adapted to ensure good storage conditions. In particular, they should be clean and dry and maintained within acceptable temperature limits. In particular, they should be clean and dry and maintained. This document aims to set out uniform statements on storage conditions. Temperature Storage Conditions For Pharmaceuticals.
From bestroom.one
Us Pharmacopeia Controlled Room Temperature bestroom.one Temperature Storage Conditions For Pharmaceuticals Storage areas should be designed or adapted to ensure good storage conditions. Find entries and build and print. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. In particular, they should be clean and dry and maintained. 4.3 storage areas should be designed or adapted to ensure good. Temperature Storage Conditions For Pharmaceuticals.
From www.akcp.com
Monitoring Pharmaceutical Storage Temperature AKCP Solutions Temperature Storage Conditions For Pharmaceuticals This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. Find entries and build and print. In particular, they should be clean and dry and maintained within acceptable temperature limits. For the purposes of this document, ‘controlled temperature storage’ is defined as the storage requirements for products not requiring. Temperature Storage Conditions For Pharmaceuticals.
From www.akcp.com
Temperature Excursion Management in Pharmaceutical Storage Temperature Storage Conditions For Pharmaceuticals This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. In particular, they should be clean and dry and maintained. Find entries and build and print. In particular, they should be clean and dry and maintained within acceptable temperature limits. Storage areas should be designed or adapted to ensure. Temperature Storage Conditions For Pharmaceuticals.