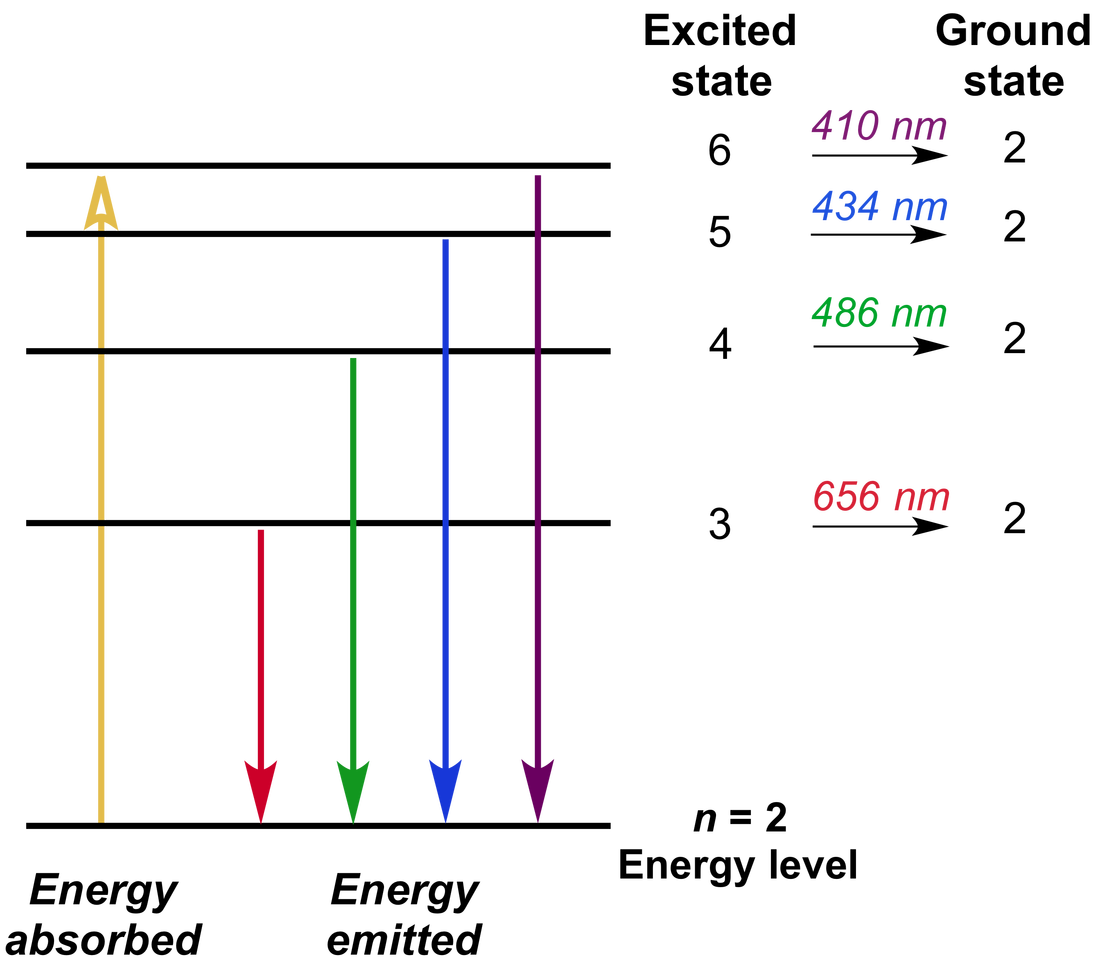
Spectra Lines Worksheet . Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. We can identify three bright lines for hydrogen in the top spectrum. Spectra samples, student worksheets, and answer key. Why are spectral lines often referred to as atomic fingerprints? Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is. How can a hydrogen atom, which has only one electron, have so many spectral lines? 4.0 (3) add to cart. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum.
from printablemediaadrianna.z13.web.core.windows.net
Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. We can identify three bright lines for hydrogen in the top spectrum. Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). How can a hydrogen atom, which has only one electron, have so many spectral lines? Spectra samples, student worksheets, and answer key. 4.0 (3) add to cart. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is. Why are spectral lines often referred to as atomic fingerprints? The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light.
Emission Spectra And Energy Levels Worksheet Answers
Spectra Lines Worksheet Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is. Why are spectral lines often referred to as atomic fingerprints? Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is. We can identify three bright lines for hydrogen in the top spectrum. How can a hydrogen atom, which has only one electron, have so many spectral lines? Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). 4.0 (3) add to cart. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. Spectra samples, student worksheets, and answer key.
From www.pinterest.com
Atomic Emission Spectrum Worksheet Printable Worksheet Template Spectra Lines Worksheet Spectra samples, student worksheets, and answer key. We can identify three bright lines for hydrogen in the top spectrum. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is. Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). Why are spectral lines often referred. Spectra Lines Worksheet.
From learningexpiamc.z21.web.core.windows.net
Waves And Spectrum Worksheets Spectra Lines Worksheet Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). We can identify three bright lines for hydrogen in the top spectrum. How can a hydrogen atom, which has only one electron, have so many spectral lines? Spectra samples, student worksheets, and answer key. 4.0 (3) add to cart. Why are spectral lines. Spectra Lines Worksheet.
From worksheets.clipart-library.com
Spectrum Worksheet Worksheet for 7th 10th Grade Spectra Lines Worksheet The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Why are spectral lines often referred to as atomic fingerprints? How can a hydrogen atom, which has only one electron, have so many spectral lines? Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different. Spectra Lines Worksheet.
From laney-lee.com
Spectrum Reading Comprehension Worksheets Spectra Lines Worksheet Why are spectral lines often referred to as atomic fingerprints? Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. We can. Spectra Lines Worksheet.
From www.worksheeto.com
16 Best Images of Atomic Spectra Worksheet Spectrum Spectra Lines Worksheet Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. How can a hydrogen atom, which has only one electron, have so many spectral lines? 4.0 (3) add to cart. We can identify three bright lines for hydrogen in the top spectrum. The emission spectrum of hydrogen is made up of lines in. Spectra Lines Worksheet.
From www.scribd.com
atomic absorption spectrum worksheet Emission Spectrum Spectroscopy Spectra Lines Worksheet Why are spectral lines often referred to as atomic fingerprints? Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). How can a. Spectra Lines Worksheet.
From www.studypool.com
SOLUTION Worksheet 2 4 spectra and electrons 2 Studypool Spectra Lines Worksheet 4.0 (3) add to cart. We can identify three bright lines for hydrogen in the top spectrum. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is. Spectra samples, student worksheets, and answer key. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of. Spectra Lines Worksheet.
From worksheetprenapetis.z14.web.core.windows.net
Waves And Spectrum Worksheets Spectra Lines Worksheet Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. How can a hydrogen atom, which has only one electron, have so many spectral lines? Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is. Why are spectral lines often referred to as atomic fingerprints?. Spectra Lines Worksheet.
From www.pinterest.ca
The Spectrum Worksheet Answers Chemistry worksheets Spectra Lines Worksheet Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Why are spectral lines often referred to as. Spectra Lines Worksheet.
From www.chegg.com
Solved Integrated Spectra Worksheet Fall 2016 Use Techniques Spectra Lines Worksheet Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). Why are spectral lines often referred to as atomic fingerprints? Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 4.0 (3) add to cart. Spectra samples, student worksheets, and answer key. Emission and absorption spectra form the basis of. Spectra Lines Worksheet.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Spectra Lines Worksheet Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. Why are spectral lines often referred to as atomic fingerprints? 4.0 (3) add to cart. Spectra samples, student worksheets, and answer key. Although objects at high temperature emit a continuous. Spectra Lines Worksheet.
From materialcampussteffen.z19.web.core.windows.net
Emission Spectra Worksheet Spectra Lines Worksheet Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). Spectra samples, student worksheets, and answer key. 4.0 (3) add to cart. How can a hydrogen atom, which has only one electron, have so many spectral lines? The emission spectrum. Spectra Lines Worksheet.
From printablemediaadrianna.z13.web.core.windows.net
Emission Spectra And Energy Levels Worksheet Answers Spectra Lines Worksheet How can a hydrogen atom, which has only one electron, have so many spectral lines? Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 4.0 (3) add to cart. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. Measuring from the scale, the wavelengths are 435 nm (purple),. Spectra Lines Worksheet.
From spiff.rit.edu
Spectrographs and Spectra Spectra Lines Worksheet Spectra samples, student worksheets, and answer key. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). How can a hydrogen atom, which has only one electron, have so many spectral lines? Why are spectral lines. Spectra Lines Worksheet.
From es.scribd.com
Atomic Spectra Worksheet Answer Key 0506 PDF Spectra Lines Worksheet 4.0 (3) add to cart. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). Why are spectral. Spectra Lines Worksheet.
From informacionpublica.svet.gob.gt
Solved The Top Spectrum On The Following Slide Was Spectra Lines Worksheet 4.0 (3) add to cart. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. Spectra samples, student worksheets, and answer key. Why are spectral lines often referred to as atomic fingerprints? The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Emission and absorption spectra. Spectra Lines Worksheet.
From www.pinterest.com
Visible light spectrum worksheet Visible light spectrum, Color Spectra Lines Worksheet Spectra samples, student worksheets, and answer key. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. Although objects. Spectra Lines Worksheet.
From www.chegg.com
Solved Worksheet Explaining Line Spectra Using the Spectra Lines Worksheet Spectra samples, student worksheets, and answer key. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is. We can identify three bright lines for hydrogen in the top spectrum. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Why are. Spectra Lines Worksheet.
From www.worksheeto.com
20 Bright Line Spectra Worksheet / Spectra Lines Worksheet Why are spectral lines often referred to as atomic fingerprints? 4.0 (3) add to cart. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is. Build and calibrate a simple spectroscope. Spectra Lines Worksheet.
From thekidsworksheet.com
Atomic Emission Spectra And The Quantum Mechanical Model Worksheet Spectra Lines Worksheet How can a hydrogen atom, which has only one electron, have so many spectral lines? Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. We can identify three bright lines for hydrogen in the top spectrum. Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). Why are spectral. Spectra Lines Worksheet.
From www.worksheeto.com
12 Emission Spectra Worksheet / Spectra Lines Worksheet Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). We can identify three bright lines for hydrogen in the top spectrum. 4.0 (3) add to cart. How can a hydrogen atom, which has only one electron, have so many spectral lines? Spectra samples, student worksheets, and answer key. Emission and absorption spectra. Spectra Lines Worksheet.
From study.com
Quiz & Worksheet Atomic Spectra Characteristics & Types Spectra Lines Worksheet Why are spectral lines often referred to as atomic fingerprints? The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. How can a hydrogen atom, which has only one electron, have so. Spectra Lines Worksheet.
From www.pinterest.com
The Spectrum Worksheet Answers 33 Waves Worksheet Spectra Lines Worksheet Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. How can a hydrogen atom, which has only one electron, have so many spectral lines? 4.0 (3) add to cart. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. We can identify three bright lines. Spectra Lines Worksheet.
From studylib.net
SPECTRA Worksheet The following IR spectrum is of a common Spectra Lines Worksheet Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is. We can identify three bright lines for hydrogen in the top spectrum. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Build and calibrate a simple spectroscope capable of measuring. Spectra Lines Worksheet.
From learningexpiamc.z21.web.core.windows.net
Waves And Spectrum Worksheets Spectra Lines Worksheet Spectra samples, student worksheets, and answer key. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. 4.0 (3) add to cart. How can a hydrogen atom, which has only one electron, have so many spectral lines? Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm. Spectra Lines Worksheet.
From dokumen.tips
(DOC) Worksheetexplaining Line Spectra DOKUMEN.TIPS Spectra Lines Worksheet Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is. 4.0 (3) add to cart. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. We can identify three bright lines for hydrogen in the top spectrum. Why are spectral lines often referred to as atomic fingerprints? The emission. Spectra Lines Worksheet.
From studylib.net
SPECTRA WORKSHEET Spectra Lines Worksheet Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. We can identify three bright lines for hydrogen in the top spectrum. Spectra samples, student worksheets, and answer key. 4.0 (3) add to cart. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the. Spectra Lines Worksheet.
From www.studocu.com
PreWorksheet 2 Absorption and Emission Spectra Revised PreWorksheet Spectra Lines Worksheet How can a hydrogen atom, which has only one electron, have so many spectral lines? The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. We can identify three bright lines for hydrogen in the top spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra. Spectra Lines Worksheet.
From dokumen.tips
(PDF) Stellar Spectra Worksheets DOKUMEN.TIPS Spectra Lines Worksheet Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. How can a hydrogen atom, which has only one electron, have so many spectral lines? Build and calibrate a simple spectroscope capable. Spectra Lines Worksheet.
From www.savemyexams.co.uk
Identifying Elements Within Stars Using Spectral Lines (5.11.3) OCR A Spectra Lines Worksheet How can a hydrogen atom, which has only one electron, have so many spectral lines? Why are spectral lines often referred to as atomic fingerprints? Spectra samples, student worksheets, and answer key. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. The emission spectrum of hydrogen is made up of lines in. Spectra Lines Worksheet.
From www.scribd.com
Mass Spectra Worksheet 1 PDF Mass Spectrometry Observational Spectra Lines Worksheet Spectra samples, student worksheets, and answer key. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. How can a hydrogen atom, which has only one electron, have so many spectral lines? The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Although objects at high. Spectra Lines Worksheet.
From printablezoneenrique.z21.web.core.windows.net
Emission Spectra Worksheet Spectra Lines Worksheet Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). 4.0 (3) add to cart. Spectra samples, student worksheets,. Spectra Lines Worksheet.
From printableschoolwhiskers.z13.web.core.windows.net
Atomic Spectra Worksheet Spectra Lines Worksheet Why are spectral lines often referred to as atomic fingerprints? Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is. 4.0 (3) add to cart. We can identify three bright lines for hydrogen in the top spectrum. Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657. Spectra Lines Worksheet.
From www.edplace.com
The Spectrum worksheet from EdPlace Spectra Lines Worksheet Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). 4.0 (3) add to cart. We can identify three bright lines for hydrogen in the top spectrum. Build and calibrate a simple spectroscope capable of measuring. Spectra Lines Worksheet.
From goc-oivf2.blogspot.com
40 emission spectra and energy levels worksheet Worksheet Information Spectra Lines Worksheet How can a hydrogen atom, which has only one electron, have so many spectral lines? We can identify three bright lines for hydrogen in the top spectrum. Why are spectral lines often referred to as atomic fingerprints? The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Although objects. Spectra Lines Worksheet.